��Ŀ����
��Դ���������Ϊ��Լ������ᾭ�÷�չ��ƿ����Խ��Խ��Ĺ��ҿ�ʼʵ�С�����ƻ���������̫������Դ��Ѱ�÷�չ���¶�����
��1��̫������ˮ���г�ʹ��һ�����������Ͻ������Ϊ���ռ���̫��������Ϳ�㣬��̬��ԭ��M���ϵ�δ�ɶԵ�����Ϊ ��
��2�������ѧ�����������л�̫���ܹ�����Ч��ͻ��5.3%�����ߴ���C60���䡰������������C60�Ľṹ��ͼ1��������̼ԭ�ӹ�����ӻ�����Ϊ ��1mol C60�����Цм�����ĿΪ ��
��3������̪ݼ������ڹ�̫���ܵ��������Ҫ���ã�һ�ֽ���þ̪ݼ�����Ľṹ��ͼ2���ýṹ�У�̼��֮��Ĺ��ۼ������� ����ԭ�ӹ���ص���ʽ��д���ۼ������ͣ�������ͼͼ2���ü�ͷ��ʾ����λ����

��4����Ԫ�����ﱡĤ̫���ܵ�ز���Ϊ���Σ�����Ҫ�����黯�ء����ӡ���п��ͭ������Ĥ��صȣ�
�ٵ�һ�����ܣ�As Se�����������������=������
����п�ľ����У��ṹ��ͼ3��ʾ���������ӵ���λ���� ��
�۶����������ӵĿռ乹��Ϊ ��
���黯�ؿ��ɣ�CH3��3Ga��AsH3��700���·�Ӧ�Ƶã���Ӧ�ķ���ʽΪ ��
��1��̫������ˮ���г�ʹ��һ�����������Ͻ������Ϊ���ռ���̫��������Ϳ�㣬��̬��ԭ��M���ϵ�δ�ɶԵ�����Ϊ
��2�������ѧ�����������л�̫���ܹ�����Ч��ͻ��5.3%�����ߴ���C60���䡰������������C60�Ľṹ��ͼ1��������̼ԭ�ӹ�����ӻ�����Ϊ
��3������̪ݼ������ڹ�̫���ܵ��������Ҫ���ã�һ�ֽ���þ̪ݼ�����Ľṹ��ͼ2���ýṹ�У�̼��֮��Ĺ��ۼ�������

��4����Ԫ�����ﱡĤ̫���ܵ�ز���Ϊ���Σ�����Ҫ�����黯�ء����ӡ���п��ͭ������Ĥ��صȣ�
�ٵ�һ�����ܣ�As
����п�ľ����У��ṹ��ͼ3��ʾ���������ӵ���λ����
�۶����������ӵĿռ乹��Ϊ
���黯�ؿ��ɣ�CH3��3Ga��AsH3��700���·�Ӧ�Ƶã���Ӧ�ķ���ʽΪ
���㣺ԭ�Ӻ�������Ų�,�����ijɼ����,�����ļ���,ԭ�ӹ���ӻ���ʽ���ӻ������ж�
ר�⣺��ѧ���뾧��ṹ
��������1����̬Niԭ�Ӻ�������Ų�ʽΪ1S22S22P63S23P63d84s2���ݴ��жϣ�
��2��ÿ��Cԭ�ӳ�3���Ҽ���û�йµ��Ӷԣ��ӻ������ĿΪ3���ݴ�ȷ���ӻ���ʽ��
���þ�̯������ÿ��̼ԭ�Ӻ��м����м����Ӷ�����1mol C60�����Цм�����Ŀ��
��3��C��Nԭ��֮���γ�˫������������λ�����ṩ�µ��ӶԵ�ԭ��ָ���ṩ�չ����ԭ�ӣ�
��4����ͬһ����Ԫ�صĵ�һ����������ԭ�����������������������ƣ�ע���VA��Ԫ�ش���ͬ��������Ԫ�صĵ�һ�����ܣ�
���ɾ����ṹ��֪��ÿ������������4��п���ӣ�������λ����4��
�۸��ݼ۲���ӶԻ�������ȷ����ռ乹�ͣ�
���黯�ؿ��ɣ�CH3��3Ga��AsH3��700���·�Ӧ�Ƶã���Ԫ���غ��֪�����ɼ��飮
��2��ÿ��Cԭ�ӳ�3���Ҽ���û�йµ��Ӷԣ��ӻ������ĿΪ3���ݴ�ȷ���ӻ���ʽ��
���þ�̯������ÿ��̼ԭ�Ӻ��м����м����Ӷ�����1mol C60�����Цм�����Ŀ��
��3��C��Nԭ��֮���γ�˫������������λ�����ṩ�µ��ӶԵ�ԭ��ָ���ṩ�չ����ԭ�ӣ�
��4����ͬһ����Ԫ�صĵ�һ����������ԭ�����������������������ƣ�ע���VA��Ԫ�ش���ͬ��������Ԫ�صĵ�һ�����ܣ�
���ɾ����ṹ��֪��ÿ������������4��п���ӣ�������λ����4��
�۸��ݼ۲���ӶԻ�������ȷ����ռ乹�ͣ�
���黯�ؿ��ɣ�CH3��3Ga��AsH3��700���·�Ӧ�Ƶã���Ԫ���غ��֪�����ɼ��飮
���
�⣺��1����̬Niԭ�Ӻ�������Ų�ʽΪ1S22S22P63S23P63d84s2��3d�����2�������ӣ��ʴ�Ϊ��2��
��2��ÿ��Cԭ�ӳ�3���Ҽ���û�йµ��Ӷԣ��ӻ������ĿΪ3������Cԭ�Ӳ���sp2 �ӻ���
ÿ��̼ԭ�Ӻ��еĦм�����Ϊ
������1mol C60�����ЦҼ�����Ŀ=1mol��60��
��NAmol-1=30NA��
�ʴ�Ϊ��sp2��30NA��
��3��C��Nԭ��֮���γ�˫��������������Ϊ�Ҽ���˫������1���Ҽ���1���м���
��λ�����ṩ�µ��ӶԵ�ԭ��ָ���ṩ�չ����ԭ�ӣ����Ը�������е���λ��Ϊ��
 ���ʴ�Ϊ���Ҽ����м���
���ʴ�Ϊ���Ҽ����м��� ��
��
��3����As��Se����ͬһ���ڣ���As���ڵ�VA�壬Se���ڵ�VIA�壬Asԭ��4p�ܼ�����3�����ӣ�Ϊ�����ȶ�״̬�������ϵͣ���һ�����ܸ���Se�����Ե�һ������As��Se���ʴ�Ϊ������
���ɾ����ṹ��֪��ÿ������������4��п���ӣ�������λ����4���ʴ�Ϊ��4��
�۶�������������Seԭ�Ӽ۲���Ӷ�=2+
=3���Һ���һ���µ��Ӷԣ�������ռ�ṹΪV�Σ��ʴ�Ϊ��V�Σ�
���黯�ؿ��ɣ�CH3��3Ga��AsH3��700���·�Ӧ�Ƶã���Ԫ���غ��֪�����ɼ��飬��Ӧ����ʽΪ����CH3��3Ga+AsH3
GaAs+3CH4 ���ʴ�Ϊ����CH3��3Ga+AsH3
GaAs+3CH4 ��
��2��ÿ��Cԭ�ӳ�3���Ҽ���û�йµ��Ӷԣ��ӻ������ĿΪ3������Cԭ�Ӳ���sp2 �ӻ���
ÿ��̼ԭ�Ӻ��еĦм�����Ϊ
| 1 |
| 2 |
| 1 |
| 2 |
�ʴ�Ϊ��sp2��30NA��
��3��C��Nԭ��֮���γ�˫��������������Ϊ�Ҽ���˫������1���Ҽ���1���м���
��λ�����ṩ�µ��ӶԵ�ԭ��ָ���ṩ�չ����ԭ�ӣ����Ը�������е���λ��Ϊ��
 ���ʴ�Ϊ���Ҽ����м���
���ʴ�Ϊ���Ҽ����м��� ��
����3����As��Se����ͬһ���ڣ���As���ڵ�VA�壬Se���ڵ�VIA�壬Asԭ��4p�ܼ�����3�����ӣ�Ϊ�����ȶ�״̬�������ϵͣ���һ�����ܸ���Se�����Ե�һ������As��Se���ʴ�Ϊ������
���ɾ����ṹ��֪��ÿ������������4��п���ӣ�������λ����4���ʴ�Ϊ��4��
�۶�������������Seԭ�Ӽ۲���Ӷ�=2+
| 6-2��2 |
| 2 |
���黯�ؿ��ɣ�CH3��3Ga��AsH3��700���·�Ӧ�Ƶã���Ԫ���غ��֪�����ɼ��飬��Ӧ����ʽΪ����CH3��3Ga+AsH3
| ||
| ||
�����������Ƕ����ʽṹ�Ŀ��飬�漰��������Ų����ӻ���������ӽṹ�����ʡ������ܡ���ѧ���������ȣ��Ѷ��еȣ������þ�̯�����о����йؼ��㣬ע����λ���γ�������

��ϰ��ϵ�д�
 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�
�����Ŀ
���¡����ݵ������¶���N2��g��+3H2��g��?2NH3��g����H��0�ķ�Ӧ���ﵽ��ѧƽ��״̬�ı�־Ϊ��������
| A���Ͽ�һ��N��N����ͬʱ��6��N-H������ |
| B�����������ܶȲ��� |
| C����������ѹǿ���� |
| D��N2��H2��NH3������֮��Ϊ1��3��2��״̬ |
���й����л����������������ǣ�������
| A����ϩ��ʹ��ˮ������KMnO4��Һ��ɫ |
| B���������Ϊ75%�ľƾ���Һ��ʹϸ�������ʱ��� |
| C��1mol�����1mol�����ڹ��������³�ַ�Ӧ������1mol CH3Cl |
| D��CH3CH2CH2CH3��CH3CH��CH3��2��Ϊͬ���칹�� |
Li-FePO4��طŵ�ʱ�ĵ�ط�ӦΪ��FePO4+Li�TLiFePO4�������Ϊ��Li+�ĵ�����壮�����й�Li-FePO4���˵����ȷ���ǣ�������
| A���ɼ�����������ߵ���ʵĵ����� |
| B�����ʱLiFePO4ֻ������ԭ��Ӧ |
| C���������У�����������ϵ��������� |
| D���ŵ�ʱ���������ӦΪ��FePO4+Li++e-�TLiFePO4 |
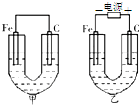 ��ͼ��ʾ���ס������صĵ缫���϶���������̼������ش�
��ͼ��ʾ���ס������صĵ缫���϶���������̼������ش�