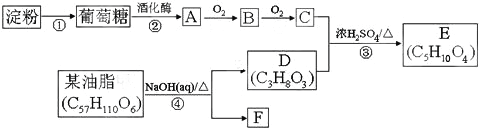
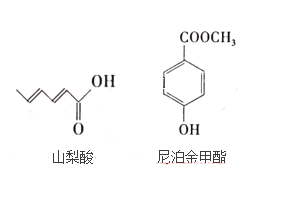
��Ŀ����
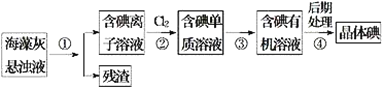
����Ŀ������������һ�ָ�Ч��������ͨ��״���¶�������������ˮ���е�Ϊ11.0�棬���ױ�ը����ȡ��ʹ�ö�������ʱҪ�������ȶ������尴һ������ϡ�ͣ��Է���ը��ijʵ��С���ڸ������ϡ�������£��ø����������������������Ʊ��������ȣ�ʵ��װ����ͼ��ʾ��
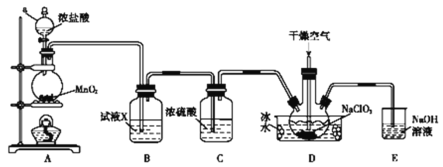
(1)����a������Ϊ___________��װ��A�з�Ӧ�Ļ�ѧ����ʽΪ___________��
(2)�Լ�X��___________��
(3)װ��D�ڷ�����Ӧ�Ļ�ѧ����ʽΪ___________��
(4)��ҵ��Ҳ���������˫��ˮ��ԭNaClO3�Ʊ�ClO2�����֮����˫��ˮ�Ʊ�ClO2�������ţ����ܵ�ԭ����_____________________________��
(5)װ��E����Ҫ��Ӧ�����ӷ���ʽΪ____________________��
(6)��֪NaClO2������Һ���¶ȵ���38��ʱ�����ľ�����NaClO2��3H2O������38��ʱ����������NaClO2������60��ʱNaClO2�ֽ��NaClO3��NaCl��
�������ʵ��������NaClO2��Һ�Ƶ�NaClO2����IJ������裺A����ѹ��55�������ᾧ��B�����ȹ��ˣ�C����38��60�����ˮϴ�ӣ�D������60�����õ���Ʒ��
��ȡ�������ò�Ʒ2.50g����ˮ���250mL��Һ��ȡ��25.00mL��Һ����ƿ�У��ټ��������ữ��KI��Һ����ַ�Ӧ��(NaClO2����ԭΪCl-�����ʲ��μӷ�Ӧ)������2��3�ε�����Һ����0.500mol��L-1Na2S2O3��Һ�ζ����յ㡣����3��ʵ���ƽ����ȥ��Һ18.80mL���Լ���NaClO2��Ʒ�Ĵ���__________(��֪��2Na2S2O3+I2=Na2S4O6+2NaI)��
���𰸡���Һ©�� ![]() ����ʳ��ˮ 2NaClO2+Cl2=2NaCl+2ClO2 H2O2����������ΪO2������Ⱦ����(��HCl����������ΪCl2����Ⱦ����) )Cl2+2OH-=Cl-+ClO-+H2O 85.07%(����0.8507����)
����ʳ��ˮ 2NaClO2+Cl2=2NaCl+2ClO2 H2O2����������ΪO2������Ⱦ����(��HCl����������ΪCl2����Ⱦ����) )Cl2+2OH-=Cl-+ClO-+H2O 85.07%(����0.8507����)
��������
������Ҫ��ʵ�����Ʊ����������ӡ����������������������ʵ�飬�����ݷ�Ӧ����������������ԭ��Ӧ��ԭ����д��Ӧ����ʽ��ͬʱ���ж���ʵ������������NaClO2��Ʒ�Ĵ��ȣ�ֻҪ����֪ʶ��ʵ�����������ѶȲ�����
(1)��ͼ�п���ֱ�ӿ�������a������Ϊ��Һ©����װ��A�о��Ƿ�����ʵ�����Ʊ������Ļ�ѧ����ʽΪ![]() ���ʴ�Ϊ����Һ©��
���ʴ�Ϊ����Һ©�� ![]() ��
��
(2)����ʵ����Ҫ��װ��B�ǽ��г��ӣ�����ȥ�����е��Ȼ������壬���Լ�X�DZ���ʳ��ˮ���ʴ�Ϊ������ʳ��ˮ��
(3)������֪��Ӧ��ΪNaClO2��Cl2����������ΪClO2���˹������ȵĻ��ϼ����ߣ���ȻҪ��Ԫ�ػ��ϼ۽��ͣ����Ƴ���һ��������ΪNaCl��Ȼ���ٽ���������ԭ��Ӧ��ƽ����װ��D�ڷ�����Ӧ�Ļ�ѧ����ʽΪ2NaClO2+Cl2=2NaCl+2ClO2���ʴ�Ϊ��2NaClO2+Cl2=2NaCl+2ClO2��
(4)������������NaClO3��һ�������·�Ӧ������������Ⱦ��������H2O2����������ΪO2������Ⱦ�������ʹ�ҵ��Ҳ���������˫��ˮ��ԭNaClO3�Ʊ�ClO2�����֮����˫��ˮ�Ʊ�ClO2�������ţ����ܵ�ԭ����H2O2����������ΪO2������Ⱦ����(��HCl����������ΪCl2����Ⱦ����)���ʴ�Ϊ��H2O2����������ΪO2������Ⱦ����(��HCl����������ΪCl2����Ⱦ����)��
(5)װ��E�ǽ���β����������NaOH��Һ����������������Ҫ��Ӧ�����ӷ���ʽΪCl2+2OH-=Cl-+ClO-+H2O���ʴ�Ϊ��Cl2+2OH-=Cl-+ClO-+H2O��
(6) ���������ữ��KI��Һ����ַ�Ӧ��(NaClO2����ԭΪCl-�����ʲ��μӷ�Ӧ�����ݵ�ʧ����������ȣ���֪![]() ��I2�����ʵ���֮��Ϊ1��2�����������ҳ����¹�ϵʽ��
��I2�����ʵ���֮��Ϊ1��2�����������ҳ����¹�ϵʽ��![]() --2I2--4
--2I2--4![]() ����
����![]()
![]()
����õ���Ʒ��NaClO2����������=![]() ���ʴ�Ϊ��85.07%(��0.8507����)��
���ʴ�Ϊ��85.07%(��0.8507����)��

 ���������������Բ��������ϵ�д�
���������������Բ��������ϵ�д�