��Ŀ����
11�����й��ڻ�ѧ��������й���������ȷ���ǣ�������| A�� | ������ë��ľ�ĺͲ������ά������Ȼ��ά������ë������˿�Ǻϳ���ά | |
| B�� | �籾���������ص������˵������⣬�����ƻ�������ķ��ȴ�����һ���±��������Ҫ�������ȵ�����������еĻ�������ԭ�� | |
| C�� | ú�ĸ�������н�̿��ú���͡���¯�����ְ�ˮ�ʹֱ��� | |
| D�� | �����ͺϳ�ϴ�Ӽ���ȥ��ԭ�����ƣ���̬�ĺϳ�ϴ�Ӽ�����ϴ�·ۣ����뵰��ø������߶�Ѫ�����̼��ȵ����������ȥ������ |
���� A������˿������ά�������ɣ�����ëҲ�����ڣ��������ںϳ���ά�ࣻ
B�����ȴ�����Ҫ�Ǻ������ȵ�������������еĻ�������ԭ�ӣ�
C��ú����������ǿ����Ҫ�õ���̿��ú���͡��ְ�ˮ�ͽ�¯����
D�������ͺϳ�ϴ�Ӽ����������ӱ�����Լ���
��� �⣺A��������ë��ľ�ĺͲ������ά������Ȼ��ά������˿������ά�������ɣ�����ëҲ�����ڣ��������ںϳ���ά�࣬��A��ȷ��
B�����ȴ��飬����һ���±���飬��Ҫ�Ǻ������ȵ�������������еĻ�������ԭ�ӣ���B��ȷ��
C��ú����������ǿ����Ҫ�õ���̿��ú���͡��ְ�ˮ�ͽ�¯����û�дֱ�����C����
D�������ͺϳ�ϴ�Ӽ����������ӱ�����Լ���ȥ��ԭ����ͬ����D��ȷ��
��ѡC��
���� ���⿼�黯ѧ������漰��ά�����ȴ��顢ú�ĸ����������Լ��ȣ������ڳ�ʶ�����ݵĿ��飬�ѶȲ���ע�����֪ʶ�Ļ��ۣ�

��ϰ��ϵ�д�
 �ܿ�����ĩ��̾�ϵ�д�
�ܿ�����ĩ��̾�ϵ�д�
�����Ŀ
1������˵����ȷ���ǣ�������
| A�� | ʯ�ͳ�ѹ�����ɵõ����͡�ú�͵������ͣ��Լ����͡�ʯ���� | |
| B�� | ���Ʊ���������ʱ�������з�Ӧ CH2=CH2+Cl2+Ca��OH��2��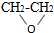 +CaCl2+H2O���˷�Ӧԭ�������ʴﵽ�� 100%�� +CaCl2+H2O���˷�Ӧԭ�������ʴﵽ�� 100%�� | |
| C�� | ú�ĸ���ú��������Һ���ܹ�ʵ��ú���ۺ����ã����������仯�����ڻ�ѧ�仯 | |
| D�� | �������Ĵ�����ˮ�����ŷţ�����ɰ�ɫ��Ⱦ |
2��NA���������ӵ�������ֵ������������ȷ���ǣ�������
| A�� | 60g�����д��ڵĹ��ۼ�����Ϊ10 NA | |
| B�� | 1L 0.1mol/L NaHCO3��Һ��HCO3-��CO32-������֮��Ϊ0.1 NA | |
| C�� | ���ڿ����п����ɶ��������23g�Ƴ��ȼ��ʱת�Ƶ�����Ϊ1 NA | |
| D�� | 235g����${\;}_{92}^{235}$U�����ѱ䷴Ӧ��${\;}_{92}^{235}$U+${\;}_{0}^{1}$n$\stackrel{�ѱ�}{��}$${\;}_{38}^{90}$Sr+${\;}_{34}^{136}$Xe+10${\;}_{0}^{1}$n�������������ӣ�${\;}_{0}^{1}$n����Ϊ10 NA |
1�����ȸʯ����Ҫ�ɷ�ΪCuCO3•Cu��OH��2��CuSiO3•2H2O��������SiO2��FeCO3��Fe2O3��Al2O3�����ʣ��Թ��ȸʯΪԭ����ȡ����ͭ�Ĺ����������£�
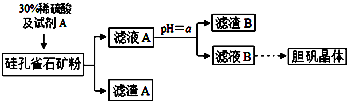
�����������↑ʼ��������ȫ������pH���±�
��ش��������⣺
��1������B����Ҫ�ɷ���Fe��OH��3��Al��OH��3���û�ѧʽ��ʾ�����жϱ�ʵ���ܷ������ҺpHʹ������ȫ��ȥ������ʧCu2+�����������ɷ�������ȫ������ʱͭ�����г���
��2�������ӷ���ʽ��ʾ�����Լ�A������2Fe2++H2O2+2H+�T2Fe3++2H2O
��3������ҺA�м���bd������ĸ����ȥ��������ʣ�
a����ˮ b������ͭ c���������� d��������ͭ
��4������ҺB����ȡ�����IJ�����������Ũ������ȴ�ᾧ�����ˡ����Ҵ�ϴ�ӡ�����ֽ���ɵȣ�
��5���ⶨ��Ʒ���Ⱥ͵����нᾧˮ��Ŀ
�ٳ������ⶨ��Ʒ����
ȡһ����������Ʒ��������ˮ������������BaCl2��Һ��ϡ���ᣬ���ˡ�ϴ�ӡ�������أ�ʵ�������ֲ�õIJ�Ʒ����ƫ�ߣ����ܵ�ԭ����acd������ĸ��
a����Ʒʧȥ���ֽᾧˮ b����Ʒ�л���CuCl2•2H2O c����Ʒ�л���Al2��SO4��3•12H2O d����Ʒ�л���Na2SO4
�ڲ�������ýᾧˮ��Ŀ
ȡag��Ʒʢװ�ڸ���������������ᾧˮȫ��ʧȥ���Ƶ���ˮ����ͭ������b g������CuSO4•nH2O����nֵ�ı���ʽΪ$\frac{80��a-b��}{9b}$��

�����������↑ʼ��������ȫ������pH���±�
| �������� | Al��OH��3 | Fe��OH��3 | Fe��OH��2 | Cu��OH��2 |
| ��ʼ������pH | 3.3 | 1.5 | 6.5 | 4.2 |
| ��ȫ������pH | 5.2 | 3.7 | 9.7 | 6.7 |
��1������B����Ҫ�ɷ���Fe��OH��3��Al��OH��3���û�ѧʽ��ʾ�����жϱ�ʵ���ܷ������ҺpHʹ������ȫ��ȥ������ʧCu2+�����������ɷ�������ȫ������ʱͭ�����г���
��2�������ӷ���ʽ��ʾ�����Լ�A������2Fe2++H2O2+2H+�T2Fe3++2H2O
��3������ҺA�м���bd������ĸ����ȥ��������ʣ�
a����ˮ b������ͭ c���������� d��������ͭ
��4������ҺB����ȡ�����IJ�����������Ũ������ȴ�ᾧ�����ˡ����Ҵ�ϴ�ӡ�����ֽ���ɵȣ�
��5���ⶨ��Ʒ���Ⱥ͵����нᾧˮ��Ŀ
�ٳ������ⶨ��Ʒ����
ȡһ����������Ʒ��������ˮ������������BaCl2��Һ��ϡ���ᣬ���ˡ�ϴ�ӡ�������أ�ʵ�������ֲ�õIJ�Ʒ����ƫ�ߣ����ܵ�ԭ����acd������ĸ��
a����Ʒʧȥ���ֽᾧˮ b����Ʒ�л���CuCl2•2H2O c����Ʒ�л���Al2��SO4��3•12H2O d����Ʒ�л���Na2SO4
�ڲ�������ýᾧˮ��Ŀ
ȡag��Ʒʢװ�ڸ���������������ᾧˮȫ��ʧȥ���Ƶ���ˮ����ͭ������b g������CuSO4•nH2O����nֵ�ı���ʽΪ$\frac{80��a-b��}{9b}$��
8������������нϹ㷺�Ŀ������ã��ṹ��ʽ��ͼ��ʾ�����ڿ��������������������ȷ���ǣ�������
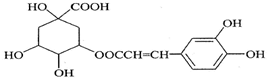
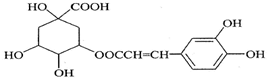
| A�� | ����ʽΪC16H18O9 | |
| B�� | 1 mol��������ˮ��ʱ������8molNaOH | |
| C�� | �뱽����ֱ̼��������ԭ�Ӷ���ͬһƽ���� | |
| D�� | ��Ũ��ˮ���ܷ���ȡ����Ӧ���ܷ����ӳɷ�Ӧ |
6�������ܱ������н��еĿ��淴Ӧ2NO2?2NO+O2������˵���ﵽƽ��״̬���ǣ�������
| A�� | ��λʱ��������a mo1 O2��ͬʱ����2a mol NO2 | |
| B�� | ��λʱ��������a mol O2��ͬʱ����2a mol NO | |
| C�� | ����������ɫ���ٸı� | |
| D�� | ��������ѹǿ���ٸı� |


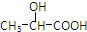 ͨ������·�߿ɺϳɣ���
ͨ������·�߿ɺϳɣ��� $��_{��}^{ŨH_{2}SO_{4}}$C3H4O2����$\stackrel{C_{2}H_{5}OH/H+}{��}$C5H8O2$��_{һ������}^{HBr}$����
$��_{��}^{ŨH_{2}SO_{4}}$C3H4O2����$\stackrel{C_{2}H_{5}OH/H+}{��}$C5H8O2$��_{һ������}^{HBr}$����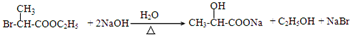 ��
�� ����Ӧ������������Ӧ����ȡ����Ӧ����
����Ӧ������������Ӧ����ȡ����Ӧ���� ��
��