��Ŀ����
17��A��B��C�����ֶ�����Ԫ�أ�����AԪ��ԭ��M���ϵ�������L����2�����ӣ�BԪ��ԭ�ӱ�AԪ��ԭ�ӵĵ��Ӳ��٣���֪BԪ����������븺���ϼ۵ľ���ֵ��ȣ�C Ԫ�ص������Ӻ�������Ų���Neԭ�Ӻ�������Ų���ͬ������֪������Ԫ�ؿ����BA2���ۻ������C2A�����ӻ���������������ش��������⣺��1��A��B��C��Ԫ�ص����Ʒֱ��ǣ�A��B̼��C�ƣ�
��2��B�����ڱ��е�λ�õڶ����ڵ�IVA�壬������C2A�ĵ���ʽ��
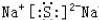 ��
����3��������BA2�Ľṹʽ�ǣ�S=C=S��
���� A��B��C�����ֶ�����Ԫ�أ�����AԪ��ԭ��M���ϵ�������L����2�����ӣ���M�������Ϊ8-2=6����AΪ��Ԫ�أ�BԪ��ԭ�ӱ�AԪ��ԭ�ӵĵ��Ӳ��٣���֪BԪ����������븺���ϼ۵ľ���ֵ��ȣ�B���ڵڶ����ڣ�����������Ϊ4����BΪ̼Ԫ�أ�C Ԫ�ص������Ӻ�������Ų���Neԭ�Ӻ�������Ų���ͬ��C���ڵ������ڣ��γ�C2A�����ӻ����CΪ+1�����ӣ���CΪNaԪ�أ��ݴ˽��
��� �⣺A��B��C�����ֶ�����Ԫ�أ�����AԪ��ԭ��M���ϵ�������L����2�����ӣ���M�������Ϊ8-2=6����AΪ��Ԫ�أ�BԪ��ԭ�ӱ�AԪ��ԭ�ӵĵ��Ӳ��٣���֪BԪ����������븺���ϼ۵ľ���ֵ��ȣ�B���ڵڶ����ڣ�����������Ϊ4����BΪ̼Ԫ�أ�C Ԫ�ص������Ӻ�������Ų���Neԭ�Ӻ�������Ų���ͬ��C���ڵ������ڣ��γ�C2A�����ӻ����CΪ+1�����ӣ���CΪNaԪ�أ�
��1��������������֪��AΪ��BΪ̼��CΪ�ƣ��ʴ�Ϊ����̼���ƣ�
��2��BΪ̼Ԫ�أ�C���ڵڶ����ڵ�IVA�壬Na2S���������������ӹ��ɣ�����ʽΪ ���ʴ�Ϊ���ڶ����ڵ�IVA�壻
���ʴ�Ϊ���ڶ����ڵ�IVA�壻 ��
��
��3��CS2������̼ԭ������ԭ��֮���γ�2�Թ��õ��Ӷԣ�����ʽΪ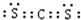 ���ṹʽΪS=C=S���ʴ�Ϊ��S=C=S��
���ṹʽΪS=C=S���ʴ�Ϊ��S=C=S��
���� ������Ҫ����ṹ����λ�ù�ϵ������ʽ�ȳ��û�ѧ����ȣ��ѶȲ����ƶ�Ԫ���ǽ���ؼ���ע�����֪ʶ���������գ�

 ȫ�ŵ�����Ԫ�ƻ�ϵ�д�
ȫ�ŵ�����Ԫ�ƻ�ϵ�д�| A�� | MgSO4 | B�� | FeCl2 | C�� | AlCl3 | D�� | NaAlO2 |
| A�� | C5H12��2��ͬ���칹�� | |
| B�� | �ױ������ϵ�һ����ԭ�ӱ���3��̼ԭ�ӵ����ȡ�����������ʹ���6�� | |
| C�� | C8H10��ֻ���������ڷ�������ͬ���칹�� | |
| D�� | CH3CH2CH2CH3��������������Ӧ��ֻ����һ��һ�ȴ��� |
| A�� | ������ | B�� | ��������ľ̿���� | ||
| C�� | �ƾ�����ˮ | D�� | HCl��������ˮ |
| A�� | �������ʱ���ȵõ�������Һ����ܻ������ڵȽӴ��������ʽ϶࣬�������� | |
| B�� | ��Һ����ʱ������Ҫ��Һ©�����Ͽڻ�������ʹ�����ϵ�С�����Ͽڲ���С����Ȼ����з�Һ | |
| C�� | ��ȡ����ʱ��Ӧѡ���л���ȡ��������ȡ�����ܶȱ����ˮ�� | |
| D�� | ��ȡ��Һ��Ҫ�õ�����ȡ�����ʣ�ͨ����Ҫ��������Ϊ�˸��õĿ����¶ȣ���ˮԡ���ȱ�ֱ�Ӽ���Ҫ�� |
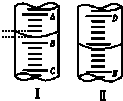 ��ͼ��ͼ���ʾ10mL��Ͳ��Һ���λ�ã�A��B��B��C�̶ȼ����1mL��ͼII��ʾ50mL�ζ�����Һ���λ�ã�D��E�̶ȼ����1mL������̶�A��D����10��������Һ��Ķ����ǣ�������
��ͼ��ͼ���ʾ10mL��Ͳ��Һ���λ�ã�A��B��B��C�̶ȼ����1mL��ͼII��ʾ50mL�ζ�����Һ���λ�ã�D��E�̶ȼ����1mL������̶�A��D����10��������Һ��Ķ����ǣ�������| A�� | ������9.2 mL��������9.40 mL | B�� | ������10.8 mL��������9.40 mL | ||
| C�� | ������9.2 mL��������10.60 mL | D�� | ������10.8 mL��������10.60 mL |
| A�� | 20g��ˮ��D2O�����еĵ�����Ϊ10 NA | |
| B�� | 46g NO2��N2O4�Ļ������������ԭ�Ӹ���Ϊ6.02��1023 | |
| C�� | 0.1mol����������������ˮ��Ӧ��ת�Ƶĵ�����Ϊ 0.2NA | |
| D�� | 1L 0.3 mol/LNa2SO4��Һ�У�����Na+��SO42-����Ϊ0.6 NA |

