��Ŀ����
8��A��B��C��D��E���ֶ�����Ԫ�ص�ԭ��������������A��Bͬ���ڣ�A��Cͬ���壬������AB2��DB2���ǿ�ʹ����ʯ��ˮ����ǵ����壮����֪CB2��E�ĵ��ʾ�������NaOH��Һ����1��д��A��B��C��D��EԪ�ص����ƣ�A̼��C�裬D��E�ȣ�
��2��д��DB2ʹ����ʯ��ˮ����ǵĻ�ѧ����ʽ��SO2+Ca��OH��2=CaSO3��+H2O��
��3��д��CB2��NaOH��Һ��Ӧ�Ļ�ѧ����ʽ��SiO2+2NaOH=Na2SiO3+H2O
��4��д��E������NaOH��Һ��Ӧ�����ӷ���ʽ��Cl2+2OH-=Cl-+ClO-+H2O��
���� A��B��C��D��E���ֶ�����Ԫ�ص�ԭ��������������A��Bͬ���ڣ�������AB2��DB2���ǿ�ʹ����ʯ��ˮ����ǵ����壬����������CO2��SO2�����֪AΪCԪ�أ�BΪOԪ�أ�DΪSԪ�أ�����֪CB2��E�ĵ��ʾ�������NaOH��Һ��E��ԭ��������������EΪCl����ϻ�ѧʽ��֪C����+4�ۣ���CΪSi���ݴ˽��
��� �⣺A��B��C��D��E���ֶ�����Ԫ�ص�ԭ��������������A��Bͬ���ڣ�������AB2��DB2���ǿ�ʹ����ʯ��ˮ����ǵ����壬����������CO2��SO2�����֪AΪCԪ�أ�BΪOԪ�أ�DΪSԪ�أ�����֪CB2��E�ĵ��ʾ�������NaOH��Һ��E��ԭ��������������EΪCl����ϻ�ѧʽ��֪C����+4�ۣ���CΪSi��
��1��������������֪��AΪ̼��CΪ�裬DΪ��EΪ�ȣ��ʴ�Ϊ��̼���裻���ȣ�
��2��SO2ʹ����ʯ��ˮ����ǵĻ�ѧ����ʽ��SO2+Ca��OH��2=CaSO3��+H2O���ʴ�Ϊ��SO2+Ca��OH��2=CaSO3��+H2O��
��3��SiO2��NaOH��Һ��Ӧ�Ļ�ѧ����ʽ��SiO2+2NaOH=Na2SiO3+H2O���ʴ�Ϊ��SiO2+2NaOH=Na2SiO3+H2O��
��4��E������NaOH��Һ��Ӧ�����ӷ���ʽ��Cl2+2OH-=Cl-+ClO-+H2O���ʴ�Ϊ��Cl2+2OH-=Cl-+ClO-+H2O��
���� ���⿼��Ԫ�ػ������ƶϣ�ע���ʹ����ʯ��ˮ����ǵ��������ֽ����ƶϣ��ѶȲ����ػ�ѧ����Ŀ��飮

| A�� | ��X+��Y2-�ĺ�����Ӳ�ṹ��ͬ����ԭ��������X��Y | |
| B�� | ��A��Ԫ�ص�ԭ�ӣ���뾶Խ����̬�⻯��Խ�ȶ� | |
| C�� | �衢�λ�ڽ�����ǽ����Ľ��紦�����������뵼����� | |
| D�� | Cs��Ba�ֱ�λ�ڵ������ڢ�A�͢�A�壬����ԣ�CsOH��Ba��OH��2 |
| ���ԭ������ | �ܶ�/��g��cm-3�� | �е�/�� | ˮ���ܽ��� | |
| ���촼 | 88 | 0.813 | 131 | �� |
| ���� | 60 | 1.0492 | 118 | �� |
| �������촼 | 130 | 0.8670 | 142 | ���� |
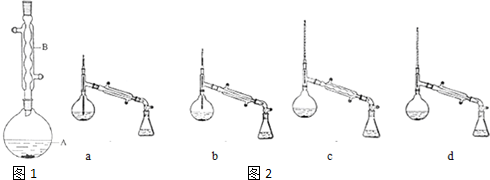
ʵ�鲽�裺
��A�м���6.6g�����촼��6.0g�����ᡢ����Ũ�����2��3Ƭ���Ƭ����ʼ��������A������50���ӣ���ӦҺ�������£�
�����ᴿ��
����ӦҺ�����Һ©���У��ֱ��ñ���̼��������Һ��ˮϴ�ӣ��ֳ��IJ������������ˮ�Ȼ��ƿ���������һ��ʱ����ȥ�Ȼ��ƣ�����ͨ�������ռ�140��143����֣�������������3.9g��
�ش��������⣺
��1��װ��B�������ǣ�����������
��2���������Ƭ�������Ƿ�ֹ���У��������һ��ʱ��������ǼӴ�Ƭ��Ӧ�ò�ȡ����ȷ������B������ȷ�𰸱�ţ���
A���������� B����ȴ�� C�����貹�� D����������
��3����ϴ�Ӳ����У��ñ���̼��������Һϴ�ӵ���ҪĿ���ǣ�ϴ����������
��4����Һ©����ʹ��ǰ����ϴ�ɾ�����©����Һ�����У�Ӧ�����Ȼ���ã����ֲ��C�����ţ���
A��ֱ�ӽ������������ӷ�Һ©���Ͽڵ���
B��ֱ�ӽ������������ӷ�Һ©���¿ڷų�
C�� �Ƚ�ˮ��ӷ�Һ©�����¿ڷų����ٽ��������������Ͽڷų�
D���Ƚ�ˮ��ӷ�Һ©�����¿ڷų����ٽ��������������¿ڷų�
��5�������ᴿ�����м�����ˮ�Ȼ��Ƶ�Ŀ����_������ˮ����
��6������������У�����ѡ��װ����ȷ������ͼ2��d�����ţ�
��7����ʵ��IJ����ǣ�40%��
| A�� | SiO2�������������������ˮҲ�������κ��� | |
| B�� | CO2ͨ��ˮ�����пɵù��� | |
| C�� | �����ʱ��SiO2��Na2CO3��Ӧ�ų�CO2������H2SiO3���Ա�H2CO3ǿ | |
| D�� | ʯӢ�����첣������Ҫԭ��֮һ�����ڳ����²���NaOH��Һ��Ӧ |
| A�� | ���ʷ����в�һ�����ڹ��ۼ� | |
| B�� | ���ӻ�����һ���������ۼ� | |
| C�� | ֻ�ɷǽ���Ԫ����ɵ�����һ���������Ӽ� | |
| D�� | ��������к��н���Ԫ�ص�һ���������Ӽ� |
| A�� | ���Ϸ�Ӧ���Ƿ��ȷ�Ӧ | |
| B�� | 2 mol��̬H2��1 mol��̬O2��������С��2 molˮ������������ | |
| C�� | ����2 mol��-�����1 mol��-�������յ������������γ�4 mol H-O�ͷŵ������� | |
| D�� | ����ȼ�չ��������仯������ͼ��ʾ |
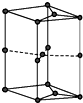 ��������и��⣺
��������и��⣺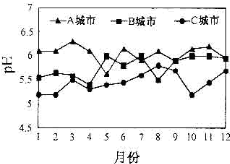 A��B��C��������ȫ����ˮ����ƽ��pH�仯��ͼ��ʾ��
A��B��C��������ȫ����ˮ����ƽ��pH�仯��ͼ��ʾ��