��Ŀ����
ʵ����Ҫ��98%���ܶ�Ϊ1��84g��cm-3������������3��68mol/L��������Һ480mL��
��1����ȷ��ȡ98%������ mL��
��2��Ҫ����3��68mol/L��������Һ480mL�������õ����������ձ�������������Ͳ
��3��������3��68mol/L��������Һ��������������ȷ�����������д����������ʹ�����Ƶ�������ҺŨ��ƫ�͵��� ��
| A����ϡ�͵�������Һת��������ƿ��δϴ���ձ��Ͳ����� |
| B�����ձ��ڵ�ϡ����������ƿ��ת��ʱ�����������ʹ����ϡ���ὦ��ƿ�� |
| C���ý�ͷ�ι�������ƿ�м�ˮʱ��Һ���������ƿ�̶ȣ���ʱ�����õιܽ�ƿ��Һ��������ʹ��Һ��Һ����̶����� |
| D���ý�ͷ�ι�������ƿ�м�ˮʱ�����ӹ۲���Һ��Һ��������ƿ�̶����� |
��1��100 ��2��500mL����ƿ ��ͷ�ι� ��3��ABCD ��4����������,��������
���������������1������Ҫ98%H2SO4�����ΪVmL��������Һϡ��ǰ����������������VmL��1��84g��cm��3��98%=0��5L��3��68mol��L��1��98g��mol��1����V=100ml��������Һ�����Ϊ100ml����Ϊ��100����2������˳���ǣ��������ȡ��ϡ�͡���ȴ����Һ�����ݡ�ҡ�ȡ�װƿ����ǩ��һ������Ͳ��ȡ���õ���ͷ�ιܣ����������ձ���ϡ�ͣ���ȴ��ת�Ƶ�500mL����ƿ�У����ò�����������ת����ϣ�����������ˮϴ���ձ���������2��3�β���ϴ��Һȫ��ת�Ƶ�����ƿ�У��ټ���������ˮ������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�ʹ��Һ�İ�Һ�����͵��������ƽ������ƿ�����������µߵ�ҡ�ȣ������������ձ�����������100mL��Ͳ��500mL����ƿ����ͷ�ιܣ���Ϊ��500mL����ƿ����ͷ�ιܣ���3��A�����ձ��ڱ�մ�����ʣ���ϡ�͵�������Һת��������ƿ��δϴ���ձ��Ͳ����������ʵ�������С��Ũ�ȼ��٣�A��ȷ��B�����ձ��ڵ�ϡ����������ƿ��ת��ʱ�����������ʹ����ϡ���ὦ��ƿ�⣬��ϡ�����к������ʣ����ʵ��������٣�Ũ�ȼ��٣�B��ȷ��C���ý�ͷ�ι�������ƿ�м�ˮʱ��Һ���������ƿ�̶ȣ���ʱ�����õιܽ�ƿ��Һ��������ʹ��Һ��Һ����̶����У���������Һ���к������ʣ����ʵ��������٣�Ũ�ȼ��٣�C��ȷ��D���ý�ͷ�ι�������ƿ�м�ˮʱ�����ӹ۲���Һ��Һ��������ƿ�̶����У���Һ��Һ����ڿ̶��ߣ���Һ�����ƫ��Ũ��ƫС��D��ȷ����4��������ʱ��һ�����ֵĴ�������������������ƣ���Ϊ���������ƣ�
���㣺����һ�����ʵ���Ũ�ȵ���Һ

 ������ϰ�ο����뵥Ԫ���ϵ�д�
������ϰ�ο����뵥Ԫ���ϵ�д� �����Ծ���ĩ���100��ϵ�д�
�����Ծ���ĩ���100��ϵ�д�þ�����������仯�������������������й㷺��Ӧ�á�
��1��þ���Ͻ����ڷɻ�����ҵ������3��90��þ���Ͻ�����������2mol/Lϡ����������0��2mol���������㲢ȷ��þ���Ͻ������ʵ���n(Mg): n(Al)= ��
��2�����������Ҫ�ɷ�ΪFeS2(��������ֻ��SiO2)�����������ԭ�ϡ�ȡij������10g�������Ŀ��������գ�4FeS2+11O2��2Fe2O3+8SO2������ַ�Ӧ����ȴ���Ƶù�������Ϊ7��4g������SiO2����Ӧ��������������FeS2����������Ϊ ��
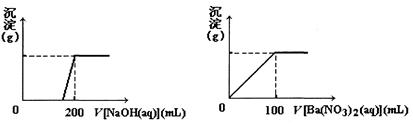
��3������һ���������ۺ�������ɵĻ�����100 mLϡ�����ַ�Ӧ����Ӧ���������κ�����ų�������Ӧ��ij�����Һ������4��00 mol��L��1��NaOH��Һ������NaOH��Һ���������������������Ĺ�ϵ��ͼ��ʾ(��Ҫʱ�ɼ��ȣ�����������ˮ�е��ܽ�)����������A�����ֵ�� ��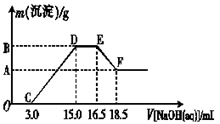
��4���������������������������֡�ȡij������ĩ28��12g(����ֻ��Fe��C)�����������г�ַ�Ӧ���õ�CO2����224mL(��״����)��
�ټ���˸�����ĩ������̼�����ʵ���֮��Ϊ ����������ȣ���
����ȡ���ݲ�ͬ����������������ĩ�ֱ�ӵ�100mL��ͬŨ�ȵ�ϡH2SO4�У���ַ�Ӧ��õ�ʵ���������±���ʾ��
| ʵ����� | �� | �� | �� |
| ���������ĩ��������g�� | 2��812 | 5��624 | 8��436 |
| ��������������L������״���� | 1��120 | 2��240 | 2��800 |
���������Һ�����ʵ���Ũ��Ϊ ��
����������ʵ����м�������m�˸�����ĩ�����㷴Ӧ������ʣ��Ĺ�������Ϊ g (����3λС��)
�ӷ���Ƕȣ�����˵����ȷ����
| A��ˮ������轺�������� |
| B��Ư��Һ��Ư�۾�����Ҫ�ɷ־�Ϊ���� |
| C��NO2��SO3���������������� |
| D�����ᡢһˮ�ϰ�������������� |
���е��뷽��ʽ������ȷ����
| A��KOH==K++OH- | B��CH3COOH==CH3COO- + H+ |
C��NH3��H2O NH4++OH- NH4++OH- | D��Na2CO3 ="=" 2Na++CO32- |