��Ŀ����
����Ŀ�������й�ͼ���˵����ȷ����

A. ͼ�ױ�ʾ����ij������Һ����μ���NaOH��Һ�����������ɳ��������ʵ�����μ�NaOH��Һ����Ĺ�ϵ
B. ͼ�ұ�ʾ�������ʵ�����NaOH��Na2CO3�Ļ����Һ����μ���ϡ��������������������������μ�HCl��Һ����Ĺ�ϵ
C. ͼ����ʾ����ϡ������Һ��������������������Һ��Fe3�����ʵ����������������ʵ����ı仯��ϵ
D. ��ȥ������������������Ȼ��ƿ��á�����Ũ�������ȹ��ˡ��ķ���
���𰸡�C
��������
A.��ij������Һ�еμ�NaOH ��Һֱ����������Һ�������ɳ�����Ȼ����������ܽ⣬ǰ�������ηֱ����ĵ�����������Һ�����֮��Ϊ3:1��ͼ����֮��������A����B.����NaOH��Na2CO3�ֱ�Ϊ1mol������1molNaOH��Na2CO3�Ļ����Һ�еμӹ�����ϡ���ᣬ���������������ᷴӦ����1molHCl��̼���������ᷴӦ����1molNaHCO3������HCl 1mol�����1molNaHCO3�������ᷴӦ����������̼���壬������HCl 1mol�� ���Բ�������ǰ��������������֮��Ϊ2:1��ͼ����֮��������B����C.��ϡ������Һ�м������ۣ��ȷ���Fe+2NO3-+4H+=Fe3++2NO��+2H2O�� ������������֮�������ӵ����ﵽ���ֵ�������������ۺ�����������������Ӧ��Fe+2Fe3+=3Fe2+�������ӵ�����Сֱ��Ϊ0������������Ӧ����������Ϊ1��0. 5=2��1����ͼ������ϣ�C��ȷ��D. KNO3�ܽ�����¶ȱ仯�ϴ��Ȼ����ܽ�����¶ȱ仯������˳�ȥ����KNO3��������NaC1���á�����Ũ������ȴ�ᾧ�����ˡ��ķ������з��룬��D����ѡC��

 ��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�
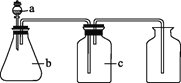
��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�����Ŀ����ͼ��a��b��c��ʾ��Ӧ�����м�����Լ���������ͼװ����ȡ���������ռ���������(����)
��� | ���� | a | b | c |
|
A | NH3 | Ũ��ˮ | ��ʯ�� | ��ʯ�� | |
B | CO2 | ���� | ̼��� | ����NaHCO3��Һ | |
C | NO | ϡ���� | ͭм | H2O | |
D | NO2 | Ũ���� | ͭм | NaOH��Һ |
A. AB. BC. CD. D