��Ŀ����
19�� ������Ԫ�� T��Q��R��W ��Ԫ�����ڱ��е�λ����ͼ��ʾ������ T �������������������� ��������ȣ����ǵ����������ˮ��������Ϊ�ס��ҡ���������������������ȷ���ǣ�������
������Ԫ�� T��Q��R��W ��Ԫ�����ڱ��е�λ����ͼ��ʾ������ T �������������������� ��������ȣ����ǵ����������ˮ��������Ϊ�ס��ҡ���������������������ȷ���ǣ�������| A�� | �ס��ҡ����������Ⱦ��ֽ� | |
| B�� | �����¶���Ũ��Һ���� T �������Ƶ�������ʢװ | |
| C�� | ����Ũ��Һ�� Q �ĵ��ʼ��ȷ�����Ӧ�������������Ϊ 1��2 ���������� | |
| D�� | R ���������ڿ������������������ÿ��γɹ⻯ѧ���� |
���� �ɶ�����Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ�ã���֪Q��R���ڵڶ����ڣ�T��W���ڵ������ڣ�T��������������������������ȣ���TΪAl������֪QΪCԪ�ء�RΪNԪ�ء�WΪSԪ�أ�
A��H2SO4��ǿ�ᣬ���ȶ������Ȳ��ֽ⣻
B�������£�����Ũ�����л�ۻ���
C������Ũ������̼���ʵķ�Ӧ��������
D���⻯ѧ�������ɵ��������ﵼ�µģ�
��� �⣺�ɶ�����Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ�ã���֪Q��R���ڵڶ����ڣ�T��W���ڵ������ڣ�T��������������������������ȣ���TΪAl������֪QΪCԪ�ء�RΪNԪ�ء�WΪSԪ�أ��ʼ�ΪAl��OH��3����ΪH2CO3����ΪHNO3����ΪH2SO4��
A��Al��OH��3��HNO3��H2CO3�����ֽ⣬H2SO4��ǿ�ᣬ���ȶ������Ȳ��ֽ⣬��A����
B�������£�����Ũ�����л�ۻ����ʳ����¶���Ũ��Һ����T�������Ƶ�������ʢװ����B��ȷ��
C��Ũ������̼���ʵķ�Ӧ��2H2SO4��Ũ��+C$\frac{\underline{\;����\;}}{\;}$2SO2��+CO2��+2H2O���ʿ����������Ϊ1��2���������壬��C��ȷ��
D���⻯ѧ�������ɵ��������ﵼ�µģ�����R�������ﵼ�µģ���D��ȷ��
��ѡA��
���� ���⿼��ṹ����λ�ù�ϵӦ�ã��ѶȲ���Ӧע��Ԫ�ص��Ƶ������Ӧ����������ˮ��������ʣ������ڿ���ѧ���Ի���֪ʶ���ۺ�Ӧ��������


| A�� | �ױ�ʾH2��O2������Ӧ�����е������仯����H2��ȼ����Ϊ241.8 kJ•mol-1 | |
| B�� | �ұ�ʾ��Na2CO3��Һ����εμ�ϡ���ᣬ����CO2�������������ʵ����Ĺ�ϵ | |
| C�� | ����ʾ��ϡ������Һ�м������ۣ���Һ��Fe3+Ũ�ȵı仯���� | |
| D�� | ����ʾt1��ʱ����������Ϊ20%�ļס���������Һ�����µ�t2��ʱ��������Һ�����ʵ�����������Ȼ��� |
| A�� | ��⾫��ͭ�Ĺ����У�ÿת��NA������ʱ�������ܽ�ͭ������Ϊ32g | |
| B�� | 1 mol Na2O2�����к���������Ϊ4NA | |
| C�� | 1mol�ǻ���17 g NH3����������֮��Ϊ9��10 | |
| D�� | 1mol Na2CO3�����к� CO${\;}_{3}^{2-}$������С��1 NA |
| A�� | ���ˮ�еμӱ���FeCl3��Һ����Һ�ʺ��ɫ��Fe3++3H2O�TFe��OH��3��+3H+ | |
| B�� | NaClO��Һ��ͨ�����SO2��ClO-+SO2+H2O�THClO+HS03- | |
| C�� | ��ͭ���缫���CuSO4��Һ��2Cu2++2H2O$\frac{\underline{\;���\;}}{\;}$2Cu+O2��+4H+ | |
| D�� | ��������Һ�еμ�����Ba��OH��2��2Al3++3SO4 2-+3Ba2++6OH-�T2Al��OH��3��+3BaSO4�� |
| A�� | 22 g 2H218O�к��е�������Ϊ10NA | |
| B�� | ���58.5 g���ڵ�NaCl���ܲ���11.2 L��������״������23.0 g������ | |
| C�� | 1.00 mol NaCl�У�����Na+��������������Ϊ10NA | |
| D�� | 1 mol Na������O2��Ӧ������Na2O��Na2O2�Ļ�����ʧȥNA������ |

| A�� | ������м��ٵ�3 g����һ���ǻ���� | |
| B�� | ��������������ٵ�����һ����Cu | |
| C�� | ���������������Եó�m��Fe2O3����m��Cu��=1��1 | |
| D�� | ���ݲ��������жϻ����X�ijɷ�ΪAl2O3��Fe2O3��Cu��SiO2 |
 ijУ��ѧ��ȤС���ͬѧ��һ��������Na2SO4��NaOH��Ʒ��NaOH�ĺ������вⶨ����ش��������⣺
ijУ��ѧ��ȤС���ͬѧ��һ��������Na2SO4��NaOH��Ʒ��NaOH�ĺ������вⶨ����ش��������⣺��1����ͬѧ���ó������ⶨ��Ʒ��NaOH�ĺ�������ͬѧѡ�õ�ҩƷ����Ʒ�⣬��Ӧ���Ȼ�����Һ��ʵ����Ӧ�ⶨ����������Ʒ�����ͳ�����������
��2����ͬѧ���õζ����ⶨ��Ʒ��NaOH�ĺ�����
���÷�����ƽ��ȡ����Ʒ5.000g��ȫ������ˮ���Ƴ�1 000.0mL��Һ���ü�ʽ�ζ�����ȡ20.00mL������Һ������ƿ�У��μӼ���ָʾ�������⣮�ζ�����ʹ��ǰ��ϴ���⣬��Ӧ��©����ϴ��
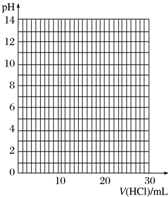
����Ũ��Ϊ0.100 0mol•L-1���������Һ���еζ�����ʼ�ζ�ǰ��һ�������ǵ���Һ���ڡ�0���̶Ȼ�0���̶����£�
�۵ζ���������pH�Ʋⶨ��ƿ����Һ��pH���ٽ��ζ��յ�ʱ�ⶨpHӦÿ��һ�β�һ�Σ�
�ܵζ������У���ƿ����Һ��pH�仯���£�
| V��HCl��/mL | 0.00 | 12.00 | 18.00 | 22.00 | 23.00 | 23.96 | 24.00 | 24.04 | 25.00 | 26.00 | 30.00 |
| pH | 13.1 | 12.6 | 12.2 | 11.7 | 11.4 | 9.9 | 7.0 | 4.0 | 2.7 | 2.4 | 1.9 |
| ָʾ�� | ��ɫ��Χ��pH�� | ��ɫ | |
| �� | �� | ||
| ���� | 3.1��4.4 | �� | �� |
| ʯ�� | 5.0��8.0 | �� | �� |
| ��̪ | 8.2��10.0 | �� | �� |
����Ʒ�У�NaOH�������ٷֺ���Ϊ96%��
��3�����µζ������ܵ������յζ����ƫ�͵���ADF
A����ʽ�ζ���ȡҺǰ���촦�����ݣ�ȡҺ��������ʧ
B���ζ����������Ӷ�ȡ��ʽ�ζ��ܵ�����
C����ƿ��ʢװ����Һ֮ǰ����������ˮ
D���ζ�ʱ����ƿ��ҡ����������Һ��ɽ�����
E����ʽ�ζ���ʹ��ǰδ��ϴ
F����ʽ�ζ���ʹ��ǰδ��ϴ��
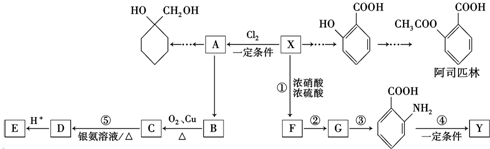
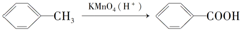
 ���������ױ�������
���������ױ�������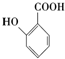 ������Ϊ���ǻ������
������Ϊ���ǻ������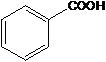 ��
��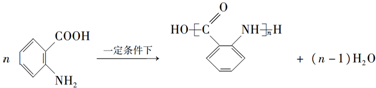 ��
��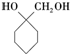 �ĺϳ�·�ߣ������Լ����ã�
�ĺϳ�·�ߣ������Լ����ã�