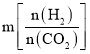��Ŀ����
����Ŀ������![]() ��
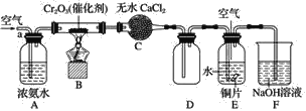
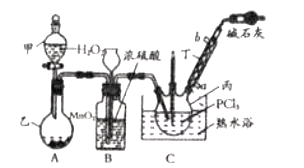
��![]() Ϊԭ�Ͽ��Ʊ��������ף����Ʊ�װ����ͼ��ʾ(�г�װ����ȥ)��
Ϊԭ�Ͽ��Ʊ��������ף����Ʊ�װ����ͼ��ʾ(�г�װ����ȥ)��
��֪![]() �����������������±���
�����������������±���
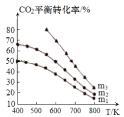
�۵� | �е� | ����������ѧ���� | |
|
|
|
|
|
|
|
(1)�����ҵ�������__________��
(2)ʵ��������������Ľ�ˮ��Ϊ__________(ѡ�![]() ����
����![]() ��)�ڡ�
��)�ڡ�
(3)װ��B��������__________(����)��
a.������� b.��עŨ���� c.�۲����������ٶ� d.������ѹ
(4)����װ�ñ�������δ���д�����и���Ӧ�Ļ�ѧ����ʽ______________________________��
(5)�Ʊ�������������PCl3�ⶨ���ȵķ������£�Ѹ�ٳ�ȡ![]() ��Ʒ��ˮ����ȫ�����
��Ʒ��ˮ����ȫ�����![]() ��Һ��ȡ��
��Һ��ȡ��![]() �������
�������![]() ����Һ����ַ�Ӧ������
����Һ����ַ�Ӧ������![]() ��Һ�ζ������ĵ⣬�յ�ʱ����
��Һ�ζ������ĵ⣬�յ�ʱ����![]() ��Һ��
��Һ��
��֪��![]() ��
��![]() ������ⶨ������û��������Ӧ�������������ݣ��ò�Ʒ��
������ⶨ������û��������Ӧ�������������ݣ��ò�Ʒ��![]() (��Է�������Ϊ137��5)�����������ļ���ʽΪ__________%��(�ú�
(��Է�������Ϊ137��5)�����������ļ���ʽΪ__________%��(�ú�![]() �Ĵ���ʽ��ʾ)
�Ĵ���ʽ��ʾ)
���𰸡�Բ����ƿ a a c d PCl3+3H2O��H3PO3��3HCl��POCl3��3H2O��H3PO4��3HCl ![]()
��������
![]() ��
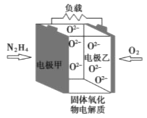
��![]() Ϊԭ�Ͽ��Ʊ��������ף�������ӦO2+2PCl3=2POCl3��װ��A������ȡ������ͨ������˫��ˮ���������Կ��Ʋ������������ʣ������к�ˮ��������Ũ�����ȥ�����������������Ȼ�����װ��C�з�Ӧ�����������ס�
Ϊԭ�Ͽ��Ʊ��������ף�������ӦO2+2PCl3=2POCl3��װ��A������ȡ������ͨ������˫��ˮ���������Կ��Ʋ������������ʣ������к�ˮ��������Ũ�����ȥ�����������������Ȼ�����װ��C�з�Ӧ�����������ס�
(1)����ͼʾװ�ã���֪�����ҵ�����ΪԲ����ƿ��
(2)������Ӧ�¶˽�ˮ���ʽ�ˮ��Ϊa��
(3)Aװ���Ƶõ������к���ˮ������װ��B��ʢ�е�Ũ�����ܳ�ȥˮ������װ��B���г���©��������ƽ��װ�������ѹǿ�����������������Ȼ���Ӧʱ��Ҫ���Ʒ�Ӧ���ʣ���ͨ���������B����Һ���۲��������١�
(4)������ʾ��֪ˮ��ķ���ʽΪ��PCl3+3H2O��H3PO3��3HCl��POCl3��3H2O��H3PO4��3HCl ��HCl�ɲ�д������š�
(5)c1mol��L1����ҺV1mL�к��еⵥ�ʵ����ʵ���Ϊ��c1 mol��L��1��V1��10-3L�� c1V1��10-3 mol�����ݷ�ӦI2��2Na2S2O3�� 2NaI��Na2S4O6��֪�������ᷴӦ���ĵĵⵥ�ʵ����ʵ���Ϊ��
c1V1��10-3 mol ![]() c2V2��10-3 mol��(c1V1
c2V2��10-3 mol��(c1V1![]() c2V2)��10-3 mol������H3PO3��H2O��I2��H3PO4��2HI��֪��25.00mL ���Ȼ���ˮ������Һ�к��е�H3PO3�����ʵ���Ϊ��n(H3PO3) =n (I2)= (c1V1
c2V2)��10-3 mol������H3PO3��H2O��I2��H3PO4��2HI��֪��25.00mL ���Ȼ���ˮ������Һ�к��е�H3PO3�����ʵ���Ϊ��n(H3PO3) =n (I2)= (c1V1![]() c2V2)��10-3 mol��250mL����Һ�к���H3PO3�����ʵ���Ϊ��(c1V1
c2V2)��10-3 mol��250mL����Һ�к���H3PO3�����ʵ���Ϊ��(c1V1![]() c2V2)��10-3 mol�� 250mL/25mL��(c1V1
c2V2)��10-3 mol�� 250mL/25mL��(c1V1![]() c2V2)��10��2 mol������m g��Ʒ�к��е����Ȼ������ʵ���Ϊ(c1V1
c2V2)��10��2 mol������m g��Ʒ�к��е����Ȼ������ʵ���Ϊ(c1V1![]() c2V2)��10��2 mol���ò�Ʒ��PCl3����������Ϊ��
c2V2)��10��2 mol���ò�Ʒ��PCl3����������Ϊ��![]() ��
��

 ����ѧ��ţ��Ӣ��ϵ�д�
����ѧ��ţ��Ӣ��ϵ�д�