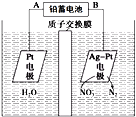
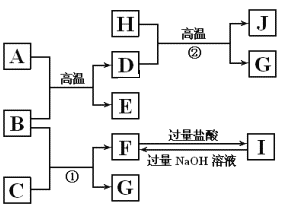
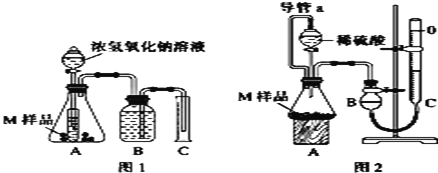
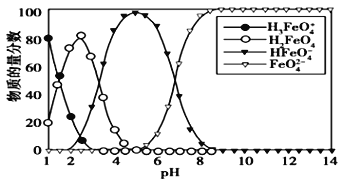
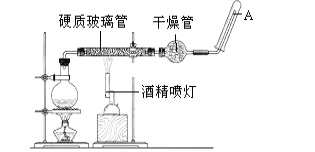
��Ŀ����
����Ŀ�������й�ʵ��ԭ���������ȷ����
A. ��20mL��Ͳ��ȡ15mL�ƾ�����ˮ5mL��������������Ϊ75%�ƾ���Һ
B. ��200mLij��������Һ�У�����1.5NA����������ӣ�ͬʱ����NA���������ӣ���������ε����ʵ���Ũ��Ϊ2.5mol��L1
C. ʵ��������2.0 mol��L1��Na2CO3��Һ950mL������ʱӦѡ�õ�����ƿ�Ĺ��ͳ�ȡNa2CO3�������ֱ�Ϊ950mL��201.4g
D. ʵ��������500 mL 0.2 mol��L1������������Һ��������ǣ�����ƽ��15.2g�̷�(FeSO4��7H2O)������С�ձ��м�ˮ�ܽ⣬ת�Ƶ�500 mL����ƿ��ϴ�ӡ�ϡ�͡����ݡ�ҡ��
���𰸡�B
��������
A����Ͳ��������������Һ��Ӧ���ձ����ƣ���15mL�ƾ�����������5mLˮ������3������A����B��1.5NA����������ӵ����ʵ���Ϊ1.5mol��NA�����������ӵ����ʵ���Ϊ1mol����������ӵĻ��ϼ�Ϊx�����ݵ���غ����֪����x=1.5��2=3���ʽ���������Ϊ+3�ۣ����Ը������ο��Ա�ʾΪM2(SO4)3������������غ����֪����������Ϊ0.5mol�����Ը������ε����ʵ���Ũ��Ϊ![]() =2.5mol��L1����B��ȷ��C������ƿû��950mL���Ӧ��1000mL������ƿ�������ƣ���m(Na2CO3)=cVM=1L��2 mol��L1��106g��mol-1=212g����C����D������500 mL 0.2 mol��L1������������Һ����Ҫ�̷�(FeSO4��7H2O)�����ʵ���=0.5L��0.2 mol��L1=0.1mol������Ϊ27.8g����D��������ѡB��
=2.5mol��L1����B��ȷ��C������ƿû��950mL���Ӧ��1000mL������ƿ�������ƣ���m(Na2CO3)=cVM=1L��2 mol��L1��106g��mol-1=212g����C����D������500 mL 0.2 mol��L1������������Һ����Ҫ�̷�(FeSO4��7H2O)�����ʵ���=0.5L��0.2 mol��L1=0.1mol������Ϊ27.8g����D��������ѡB��

����Ŀ����ϵΪԪ�����ڱ��е���B�塢ԭ������Ϊ57~71��Ԫ�ء�
��1����(Dy)�Ļ�̬ԭ�ӵ����Ų�ʽΪ[Xe]4f106s2��������(Dy)ԭ����Χ�����Ų�ͼ��___________��
��2�����³��������籵ͭ�������к���Cu3+����̬ʱCu3+ �ĵ����Ų�ʽΪ________________��
��3���۲�����������ϵԪ�صĵ������������ж����п�����ʾ+3 �۵�Ԫ����___________(��Ԫ������)��������ϵԪ�صĵ�����(��λ��kJ mol-1)
Ԫ�� | I1 | I2 | I3 | I4 |
Yb(� | 604 | 1217 | 4494 | 5014 |
Lu(�壩 | 532 | 1390 | 4111 | 4987 |
La(�磩 | 538 | 1067 | 1850 | 5419 |
Ce(�棩 | 527 | 1047 | 1949 | 3547 |
��4��Ԫ����(Ce)�����γ������(NH4)2[Ce(NO3)6]��
���������������Ԫ�����縺���ɴ�С��˳��Ϊ________________(��Ԫ�ط��ű�ʾ)��
��д�����������̬�⻯��ˮ��Һ�д��ڵ������__________________(��дһ��)��
��Ԫ��Al Ҳ�����Ƴɼ��������̬�Ȼ������ӱ�ʾΪ(AlCl3)2��������Al ԭ���ӻ���ʽΪ_____________��������������ѧ��������______________(����ĸ)��
a.���Ӽ� b.���Լ� C.�Ǽ��Լ� d.��λ��
��5��PrO2(��������)�ľ���ṹ��CaF2��������������ԭ��λ�����ĺͶ�������PrO2(��������)�ľ�������________����ԭ������֪��������Ϊa pm���ܶ�Ϊ�� g�� cm-3��NA=_____________ (�ú�a�����Ĵ���ʽ��ʾ)��