��Ŀ����
����Ŀ����ѧ�о����֣����ֽ��������ֽ�������������塣ij������ƷM����������ͭ�����е����ֻ�������ɡ�
��ʵ��Ŀ�ģ�̽�������ijɷ�
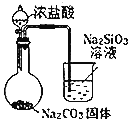
��������裩����1��M�ijɷ�������ͭ��
����2��M�ijɷ�����������
����3��M�ijɷ���______��
����4��M�ijɷ�������ͭ����
�����ʵ�飩ȡ����������Ϊm g��M��Ʒ����ͼ1��ͼ2װ�÷ֱ����ʵ�飺ʵ��ǰ���������dz��¡���ѹ������ƷM��ַ�Ӧ��ͼ1��ͼ2ʵ�鷽�����ʵ�鲢�����������ֱ�ΪV1L��V2L�����ⶨ������������ۺϳɱ�״������
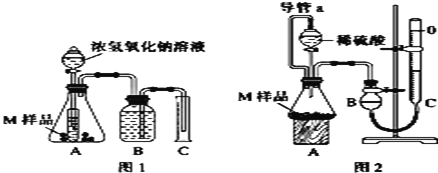
��1�����ʵ��Ŀ�ĺͲ���������ݡ�________
��2������ʵ����Ҫ0.50mol/LNaOH��Һ240mL���ù����������ʱӦ����____g NaOH����������ƽ������������NaOH�����л���Na2O���ʻᵼ��������ҺŨ��_____���ƫ����ƫС������Ӱ�족��
��3������ʵ��1ǰ��Bƿ�е�ˮû��װ����ʹ��õ��������_______���ƫ����ƫС������Ӱ�족����ʵ��2�еĵ���a������Ϊ_____________���ܷ�ֹ�����Һ����ų���ƿ�ڲ��ֿ��������²ⶨ���������ƫ��
��4����V1��0�����Ʋ���������________���1������2������3����4����һ����������������3��������V1________V2�����������������������
��5����V1��V2��0����M��Ʒ����������Ϊ____________g���ú�V1��V2ʽ�ӱ�ʾ��
���𰸡�����ͭ 5.0g ƫ�� ��Ӱ�� ƽ��ѹǿʹ����˳������ 1 = 56��V2-V1��/22.4g��2.5��V2-V1��g
��������
��1������������裬�Ƴ�����3��M�ijɷ�������ͭ��
��2��m(NaOH)=250��10��3L��0.50mol��L��1��40g��mol��1=5.0g��62gNa2O����2mol��NaOH��ÿ31gNa2O�ܻ��1molNaOH����NaOH�����л���Na2O���ʵ���������ҺŨ��ƫ��
��3�����ʵ��ǰBƿҺ��û��װ��ˮ����Ӱ��ʵ��������Ϊ������B���ռ������������������C����Һ��������ʵ��2�е���a������Ϊƽ��ѹǿ��ʹ����˳�����£�
��4����ΪV1������0��˵�����������������NaOH��Һ��Ӧ��������Ľ�����Al��������1������������3Ϊ����ͭ��ͭ����NaOH��ϡ���ᷴӦ��Al��NaOH��Ӧ��2Al��2NaOH��2H2O=2NaAlO2��3H2����Al��ϡ���2Al��3H2SO4=Al2(SO4)3��3H2������ͬ������Al����������������ͬ����V1=V2��
��5��������2������V2��V1��Ϊ�������ᷴӦ���ɵ��������������ʵ�����Ϊ![]() ��
��

 �����������Ů��ͯ������ϵ�д�
�����������Ů��ͯ������ϵ�д�