��Ŀ����
17��ʵ������������������Ʊ��������������й����ʵ�����������±���| ������ | ��Է������� | �ܶ�/g/cm3 | �е�/�� | �ܽ��/100gˮ |
| ������ | 74 | 0.80 | 118.0 | 9 |
| ������ | 60 | 1.045 | 118.1 | ���� |
| ���������� | 116 | 0.882 | 126.1 | 0.7 |
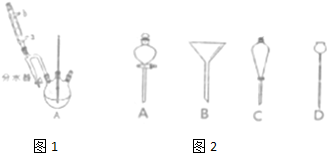
�������£�
����50mL������ƿ�У�����18.5mL��������13.4mL�����ᣬ3��4��Ũ���ᣬͶ���ʯ����װ��ˮ�������ã�ʵ������в��Ϸ����ȥ��Ӧ���ɵ�ˮ�����¶ȼƼ����������ܣ�����ͼ1��
�ڽ���ˮ���ֳ�������ͷ�ӦҺһ�����Һ©���У�ˮϴ��10%Na2CO3ϴ�ӣ���ˮϴ�����ת������ƿ�����
�۽�����������������������ƿ�У���ѹ�����ռ���֣���15.1g������������
��ش��й����⣺
��1����ˮӦ�ô�������a����a��b���˹ܿ�ͨ�룮
��2������A�з�����Ӧ�Ļ�ѧ����ʽΪCH3COOH+CH3CH2CH2CH2OH
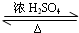 CH3COOCH2CH2CH2CH3+H2O��
CH3COOCH2CH2CH2CH3+H2O����3������١����Ϸ����ȥ��Ӧ���ɵ�ˮ���ò�����Ŀ���ǣ�ʹ�÷�ˮ�������ˮ��ʹƽ�������ƶ�����߷�Ӧ���ʣ�
��4��������У���10%Na2C03��Һϴ���л��㣬�ò�������Ŀ���dz�ȥ��Ʒ�к��е���������ʣ�
��5�����з�Һ����ʱ��ʹ�õ�©����C����ѡ�������ͼ2��
��6��������ڽ����������ʱ������118�濪ʼ�ռ���֣�����ƫ�ߣ���ߡ����ߡ��͡���ԭ���ǻ��ռ�������δ��Ӧ�ı��������������
���� ��1���������õ������������ܣ�ˮ���¿ڽ��Ͽڳ���
��2������������Ӧԭ�������ǻ�������д����Ӧ�Ļ�ѧ����ʽ��
��3��������������Ի�ѧƽ���Ӱ�������
��4�����ᶡ�������ڱ���̼������Һ��������̼���Ʒ�Ӧ�������գ�
��5����Һ����ʱ�²�Һ����¿ڷų����ϲ�Һ����Ͽڵ�����
��6������118��㿪ʼ�ռ���ִ�ʱ�������к��д���������������ռ�������δ��Ӧ�ı�����������������»�õ���������������ƫ��
��� �⣺��1��Ϊ�������Ч����ˮӦ���¿ڽ��Ͽڳ����ʴ�Ϊ��a��
��2��������Ӧ�ı���Ϊ�����ǻ��������⣬�����붡����Ũ���������¼��ȷ���������Ӧ�������ᶡ����ˮ���÷�ӦΪ���淴Ӧ����ѧ����ʽΪ��CH3COOH+CH3CH2CH2CH2OH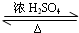 CH3COOCH2CH2CH2CH3+H2O��
CH3COOCH2CH2CH2CH3+H2O��
�ʴ�Ϊ��CH3COOH+CH3CH2CH2CH2OH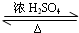 CH3COOCH2CH2CH2CH3+H2O��
CH3COOCH2CH2CH2CH3+H2O��
��3�������������Ũ�ȣ�ƽ��������Ӧ�����ƶ���ʹ�÷�ˮ�������ˮ��������ƽ��������Ӧ�����ƶ�����߷�Ӧ���ʣ�
�ʴ�Ϊ��ʹ�÷�ˮ�������ˮ��ʹƽ�������ƶ�����߷�Ӧ���ʣ�
��4���Ʊ����ᶡ��ʱ���ñ���̼������Һ��Ŀ�����кͻӷ����������ᣬʹ֮ת��Ϊ����������ˮ�У����������ᶡ������ζ���ܽ�ӷ��������Ҵ����������ᶡ����ˮ�е��ܽ�ȣ����ڷֲ�õ�����
�ʴ�Ϊ����ȥ��Ʒ�к��е���������ʣ�
��5����Һ����ʱ��ʹ�õ�©���Ƿ�Һ©������Һ©�����²�Һ����¿ڷų����ϲ�Һ����Ͽڵ�������������Һ�����Ⱦ��
�ʴ�Ϊ��C��
��6������118��㿪ʼ�ռ���ִ�ʱ�������к��д���������������ռ�������δ��Ӧ�ı����������������˻ᵼ�²���ƫ�ߣ�
�ʴ�Ϊ���ߣ����ռ�������δ��Ӧ�ı��������������
���� ������Ҫ�����������������Ʊ������������������Ʊ�ԭ���Լ�����ԭ������ʵ��װ���ǽ��Ĺؼ�����Ŀ�Ѷ��еȣ�������������ѧ���ķ������������������Ӧ����ѧ֪ʶ��������

 �����Ծ���ĩ���100��ϵ�д�
�����Ծ���ĩ���100��ϵ�д� ˫��ͬ������ѵ��ϵ�д�
˫��ͬ������ѵ��ϵ�д� �Ƹ�С״Ԫͬ������������ϵ�д�
�Ƹ�С״Ԫͬ������������ϵ�д�| A�� | CuCl2 | B�� | BaCl2 | C�� | HNO3 | D�� | NaOH |
| A�� | 25 | B�� | 53 | C�� | 78 | D�� | 131 |
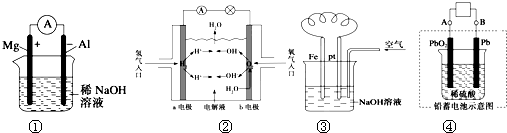
| A�� | ͼ����Ƭ�����ĵ缫��Ӧʽ�ǣ�Al+4OH--3e�TAlO2-+2H2O | |
| B�� | ͼ��b�缫�ĵ缫��ӦΪ��4OH--4e-�T2H2O+O2�� | |
| C�� | ͼ����Һ�з����˱仯��4Fe��OH��2+O2+2H2O�T4Fe��OH��3 | |
| D�� | ͼ�ܳ��ʱ��������Ӧ��PbSO4+2H2O-2e-�TPbO2+SO42-+4H+ |
| A�� | �������Ż�����ĭ��������� | |
| B�� | ʵ��̨�ϵľƾ��������Ż�������ʪĨ������ | |
| C�� | Ƥ������ŨHNO3�������ô���ˮ��ϴ������С�մ�ˮϴ�� | |
| D�� | ��������棬Ӧ��������۸��� |
| A�� | ���顢���Ȼ�̼ | B�� | ���͡����� | ||
| C�� | �Ҵ������� | D�� | 1��2-�������顢���Ȼ�̼ |
��Al ��Al2O3��AlCl3 ��Al��OH��3��
| A�� | �٢ڢ� | B�� | �ڢۢ� | C�� | �٢ۢ� | D�� | �٢ڢ� |
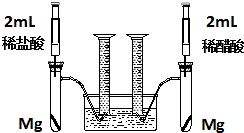 ij��ѧС��Ϊ�Ƚ�����ʹ�������ԣ����������ʵ�鷽����װ����ͼ��
ij��ѧС��Ϊ�Ƚ�����ʹ�������ԣ����������ʵ�鷽����װ����ͼ��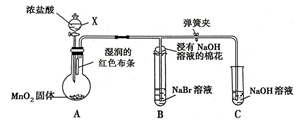 ��ͼ��һ����ȡ��������֤����ijЩ���ʵ�װ�ã����гֺͼ���װ��ʡ�ԣ�
��ͼ��һ����ȡ��������֤����ijЩ���ʵ�װ�ã����гֺͼ���װ��ʡ�ԣ�