��Ŀ����
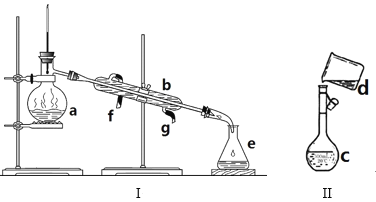
����Ŀ��ͼ�ǹ�ҵ�����ϵ��Ȼ���ϳ�������д���������ʵĻ�ѧʽ��
��1��B________��C________��
��2����ҵ���Ʊ�HC1�Ļ�ѧ����ʽ��____________��
��3��ʵ���ҳ���NaCl��Ũ������Ӧ��ȡ�Ȼ��⣬����ʱ������װ��Ӧѡ������װ�õ�________�����ţ�
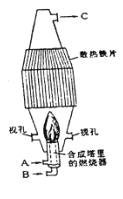
��4�����¸���β������װ���У��ʺ�������HCl���壬�����ܷ�ֹ��������________��
��5��ͼΪʵ����ijŨ�����Լ�ƿ��ǩ�ϵ��й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺

��6����Ũ������HC1�����ʵ���Ũ��Ϊ________mol/L.
��7��ȡ����������ĸ�����ʱ�������������в�����ȡ����Ķ��ٶ��仯����________
A.��Һ��HC1�����ʵ���
B.��Һ��Ũ��
C.��Һ��Cl-����Ŀ
D.��Һ���ܶ�
��8��ijѧ����������Ũ���������ˮ����500mL���ʵ���Ũ��Ϊ0.40mol/L��ϡ���ᡣ��ѧ����Ҫ��ȡ________mL����Ũ����������ơ�
��9�����в����У�ʹ��Һ��Ũ��ƫ�͵IJ�����________
A.ҡ�Ⱥ�Һ���Ե��ڿ̶��ߣ��ټ�ˮʹ֮��ƽ
B.ת��ʱ����������Һ����ʵ������
C.��Һת��ǰ������ƿ���Ѿ�����������ˮ
D.����ʱ����С�ļ�ˮ�����˿̶��ߣ��û�δҡ�ȣ��������������ˮ
���𰸡����� �Ȼ��� H2+Cl2![]() 2HCl E BD 11.9 BD 16.8 ABD
2HCl E BD 11.9 BD 16.8 ABD
��������
(1)-(2)������������ȼ�գ����������Ȼ��⣬��Ӧ�ķ���ʽΪ��H2+Cl2![]() 2HCl�����������ж�����ͨ��ܾͻ�й©����Ⱦ��������Ҫ��ȫ��Ӧ�������������ݴ˷�����
2HCl�����������ж�����ͨ��ܾͻ�й©����Ⱦ��������Ҫ��ȫ��Ӧ�������������ݴ˷�����
��3�����ڹ����Һ����Ӧ��Ҫ�����Ʊ����壬�ݴ��ص�ѡ�����õ�װ�ã�
��4�����ݼ��ܱ�֤������ȫ�����ܷ�ֹ�����������з�����
(6)����c=1000�Ѧ�/M����Ũ������HCl�����ʵ���Ũ�ȣ�
(7)��Һ�Ǿ��ȵģ���Һ���ܶȡ�Ũ�Ȳ�������仯��
(8)����ϡ�Ͷ���,ϡ��ǰ��HCl�����ʵ������䣬�ݴ˼�����ҪŨ����������
(9)�����������������ʵ�������Һ�����Ӱ�죬����c=n/V�ж϶�������ҺŨ�ȵ�Ӱ�졣
��1��������������ȼ�գ����������Ȼ��⣬��Ӧ�ķ���ʽΪ��H2+Cl2![]() 2HCl�����������ж�����ͨ��ܾͻ�й©����Ⱦ��������Ҫ��ȫ��Ӧ�����AΪ������BΪ������CΪ�Ȼ��⣻
2HCl�����������ж�����ͨ��ܾͻ�й©����Ⱦ��������Ҫ��ȫ��Ӧ�����AΪ������BΪ������CΪ�Ȼ��⣻
�ʴ��ǣ��������Ȼ��⣻
��2��������������ȼ�գ����������Ȼ��⣬��Ӧ�ķ���ʽΪ��H2+Cl2![]() 2HCl��
2HCl��
�ʴ��ǣ�H2+Cl2![]() 2HCl��
2HCl��
��3��ʵ���ҳ���NaCl��Ũ������Ӧ��ȡ�Ȼ��⣬���ڹ����Һ����Ӧ��Ҫ�����Ʊ����壬��˿���ѡ�õ�װ��ΪE��
�ʴ��ǣ�E��
��4��A����ܿ�δ����ˮ�У���Ȼ�ܹ���ֹ�����������ܳ�ֽ���β�������գ���A�����
B������ܲ���ˮ�У�����β�����գ�����U����ܵĴ��ڣ�ˮ���ѵ�����֮ǰ��װ���У���B����ȷ��
C�β��������Ȼ����ȫ�����Ǽ�������������C�����
D�����ܲ���ˮ�У�����β�����գ����ͽṹ���ڷ�ֹ��������D����ȷ��
�ʴ�ΪBD��
(6)��Ũ������HCl�����ʵ���Ũ��Ϊ1000��1.19��36.5%/36.5=11.9mol/L��
����:11.9��
(7)��Һ�Ǿ��ȵģ���Һ���ܶȡ�Ũ�Ȳ�������仯����HCl�����ʵ�����Cl-����Ŀ����Һ����йأ�
����:BD��
(8)����ҪŨ��������ΪV mL������ϡ�Ͷ��ɣ�ϡ��ǰ��HCl�����ʵ������䣬��:VmL��11.9mol/L=500mL��0.40mol/L������ó�:V=16.8��
����:16.8��
��9��A.ҡ�Ⱥ�Һ���Ե��ڿ̶��ߣ�����������������ҺŨ��ȷ������Ӱ�죻���ټ�ˮʹ֮��ƽ����Һ���������Ũ��ƫ�ͣ�
B.ת��ʱ����������Һ����ʵ�����ϣ��������ʲ������,���ʵ����ʵ���ƫС����ҺŨ��ƫ�ͣ�
C.��Һת��ǰ������ƿ���Ѿ�����������ˮ�����ջ���Ҫ��ˮ���̶��ߣ���˲�Ӱ�����ʺ���Һ�������Ũ�Ȳ��䣻
D.����ʱ����С�ļ�ˮ�����˿̶��ߣ��û�δҡ�ȣ��������������ˮ�����ʵ�����С����Һ��Ũ��ƫ�ͣ�
�ʴ�ѡABD��

 Сѧѧϰ�ð���ϵ�д�
Сѧѧϰ�ð���ϵ�д� Сѧͬ�����������ܾ�ϵ�д�
Сѧͬ�����������ܾ�ϵ�д�����Ŀ����һ�����İ�������粒�������ij�ݻ��㶨����������У�������Ӧ��H2NCOONH4(s)![]() 2NH3(g)+CO2(g)�ڲ�ͬ�¶��£��÷�Ӧƽ��״̬�������ݼ��ұ�������˵����ȷ����
2NH3(g)+CO2(g)�ڲ�ͬ�¶��£��÷�Ӧƽ��״̬�������ݼ��ұ�������˵����ȷ����
�¶� | ƽ��Ũ��/(mol��L-1) | |
c(NH3) | c(CO2) | |
T1 | 0.1 | |
T2 | 0.1 | |
A. ��T2>T1����÷�Ӧ����H<0
B. T1��T2ʱ��H2NCOONH4ת������n(T2)![]() 2��n(T1)
2��n(T1)
C. NH3�����������ʱ��˵���÷�Ӧ�ﵽƽ��
D. ���������N2��H2NCOONH4��������
����Ŀ��Ӱ�컯ѧ��Ӧ���ʵ����غܶ࣬ij��ѧС����ʵ��ķ�������̽����
I��̽���һ��
��ѡҩƷ����Ƭ��пƬ��0.5mol/LH2SO4��1.5mol/LH2SO4��18.4mol/LH2SO4
��ͬѧ�о���ʵ�鱨��
ʵ�鲽�� | ���� | ���� |
�ٷֱ�ȡ�������1.5mol/L����������֧�Թ��У� ��_____________________�� | ��Ӧ���ʣ� п>�� | ����������Խ���ã���Ӧ����Խ�� |
(1)��ͬѧʵ�鱨���е�ʵ�鲽���Ϊ__________________________________��
(2)��ͬѧ��ʵ��Ŀ����_______________________________��Ҫ�ó���ȷ��ʵ����ۣ�������Ƶ�ʵ��������__________________��
��ͬѧΪ�˶����о�Ũ�ȶԻ�ѧ��Ӧ���ʵ�Ӱ�죬������ͼ��ʾװ�ý���ʵ�飺

(3)��ͬѧ��ʵ������Ҫ�ⶨ��������_________________________��
(4)��ͬѧ����ѡ��___________mol/L������ɸ�ʵ�飬������_________________��
II��̽�������
��ѡҩƷ��0.1mol/LNa2S2O3��Һ��0.2mol/LNa2S2O3��Һ��0.1mol/LH2SO4��Һ��0.2mol/LH2SO4��Һ��
��֪��Na2S2O3��H2SO4��Na2SO4��S����SO2����H2O
ʵ�� ��� | Na2S2O3���� | H2SO4���� | �¶ȣ����� |
�� | 0.1mol/L5mL | 0.1mol/L5mL | 10 |
�� | 0.2mol/L5mL | 0.2mol/L5mL | 25 |
�� | 0.1mol/L5mL | 0.1mol/L5mL | 25 |
�� | 0.1mol/L5mL | 0.1mol/L5mL | 40 |
(1)����̽���¶ȶԻ�ѧ��Ӧ���ʵ�Ӱ�죬��ѡ��ʵ������___________��
(2)����̽��Ũ�ȶԻ�ѧ��Ӧ���ʵ�Ӱ�죬��ѡ��ʵ������___________��
(3)�ڸ�ʵ������У���Ҫ�۲�ͼ�¼________________�����Ƚϻ�ѧ��Ӧ���ʵĿ�����
(4)Na2S2O3�ڼ�����Һ�пɱ�I2����ΪNa2SO4��д���÷�Ӧ�����ӷ���ʽ___________________��
����Ŀ����1������ͼ��ʾ�������ݻ�Ϊ���ҵ��������¶���ͬ���ֱַ�����ͼ��ʾ�����������壬ͬʱ���������������ʹ�����������ַ�Ӧ�ﵽƽ�⣬������������Ӧ�ٴδﵽƽ�⣬����˵����ȷ����_____

A����һ��ƽ��ʱ��SO2�����ʵ������Ҹ���
B��ͨ������δ��Ӧǰ������ѹǿ������һ����
C����һ��ƽ��ʱ��������ѹǿһ��С������
D���ڶ���ƽ��ʱ��SO2�������ʵ����ȵ�һ��ƽ��ʱ����SO2�����ʵ�����2����Ҫ��
��2��NH3�ϳɳ����Ļ������ء���ѧʽΪCO(NH2)2����Ϊ���������е�һ��Ϊ��2NH3(g)+CO2(g)![]() NH2COONH4(s) ��H= -159.5kJ/mol�����������Ӧ����2L�����ܱ������г���2molNH3��1molCO2��ƽ��ʱ�ų�127.6kJ������������Ӧ�¶Ȳ��䣬�ڸ������г���2.8molNH3��1.4molCO2������ƽ��ʱ��c��NH3��Ϊ___________��
NH2COONH4(s) ��H= -159.5kJ/mol�����������Ӧ����2L�����ܱ������г���2molNH3��1molCO2��ƽ��ʱ�ų�127.6kJ������������Ӧ�¶Ȳ��䣬�ڸ������г���2.8molNH3��1.4molCO2������ƽ��ʱ��c��NH3��Ϊ___________��
��3���������£���0.5mol/L������Һ�м�������ˮ����ˮ�������c(H+)��c(OH��)_________�������������������С��������������
����֪Ksp��Ag2CrO4��=1.0��10��12����0.2mol/L��AgNO3��Һ�м���������0.00008mol/LK2CrO4��Һ������Һ�е�c(CrO42��)=___________��
�������£�0.1mol/LNaHCO3��Һ��pHֵ______0.1mol/LNa2SO3��Һ��pHֵ������>������<������=������֪��
H2CO3 | K1=4.3��10��7 | K2=5.6��10��11 |
H2SO3 | K1=1.54��10��2 | K2=1.02��10��7 |
��4����һ�ֿɳ����Na��Al/FeS����ع���ʱNa+�����ʵ������ֲ��䣬�������ú�Na+�ĵ��������Ϊ����ʣ���֪�õ��������ӦʽΪ2Na++FeS+2e��=Na2S+Fe����õ���ڳ��ʱ������������Ӧ��������____________���ŵ�ʱ������ӦʽΪ__________________��