��Ŀ����
��֪31g���ף�P�����壩��������ȼ������P4O10����ų�738.5kJ������31g���ף�P4�����壩��������ȼ������P4O10����ų�745.8kJ�����������ж���ȷ���� �� ��
| A��������������ȼ�յ��Ȼ�ѧ����ʽ�ǣ�P4��s��+5O2��g��=P4O10��s�� ��H=-745.8kJ��mol-1 |
| B������ת���ɰ���ʱ�ų����� |
| C��31g�������̺�������Ϊ738.5k |
| D�����ױȰ����ȶ� |
D
���������������������ȼ�յ��Ȼ�ѧ����ʽ�ǣ�P4(s)+5O2(g)=P4O10(s) ��H=-2983.2kJ��mol-1��A����ͬ�����ĺ���ȼ�շų�������С�ڰ���ȼ�շų���������˵�����������ͣ��ȶ�������ת��Ϊ������Ҫ���ȣ�B����D��ȷ������ȼ�շų������������ں����̺���������C����ѡD��
�����������ص㿼��ѧ���ķ����������ѶȽϴ�

��ϰ��ϵ�д�
�����Ŀ
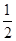 H2SO4(Ũ)+ NaOH(aq)=
H2SO4(Ũ)+ NaOH(aq)=
