题目内容
【题目】硼酸( H3BO3) 为一元弱酸,H3BO3可以通过电解的方法制备。其工作原理如右图所示( 阳膜和阴膜分别只允许阳离子、阴离子通过)。下列说法错误的是
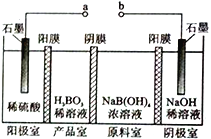
A. a 与电源的正极相连接
B. 阳极的电极反应式为:2H2O-4e-=O2↑+4H+
C. [B(OH)4]-穿过阴膜进入产品室,Na+穿过阳膜进入阴极室
D. 当电路中通过3mol 电子时,可得到1molH3BO3
【答案】D
【解析】A.与 a 极相连的石墨所处的区域为阳极室,则a与电源的正极相连接,故A正确;B. 阳极上发生氧化反应,溶液中水失去电子生成氧气,电极反应式为:2H2O-4e-=O2↑+4H+↑,故B正确;C. 在电解池中,阴离子向阳极运动,阳离子向阴极运动,因此[B(OH)4]-穿过阴膜进入产品室,Na+穿过阳膜进入阴极室,故C正确;D. 阳极电极反应式为:2H2O-4e-=O2↑+4H+,阴极上发生还原反应,溶液中的水得到电子生成氢气,2H2O+2e- = H2↑+ 2OH―,[B(OH)4]-穿过阴膜进入产品室,与氢离子反应生成H3BO3,[B(OH)4]-+ H+= H3BO3+ H2O,当电路中通过3mol 电子时,生成3mol氢离子,可得到3mol H3BO3,故D错误;故选D。

练习册系列答案
相关题目