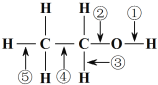
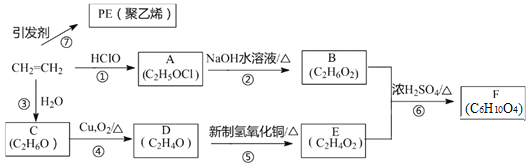
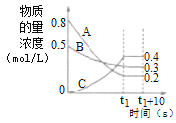
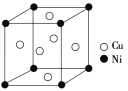
��Ŀ����
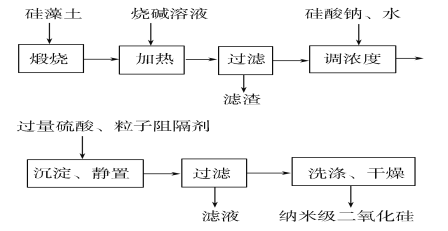
����Ŀ����������������SiO2��ɵģ���������Fe2O3��Al2O3���л�������ʣ�ͨ����dz��ɫ��dz��ɫ��������ס���ҵ�Ͽɰ���ͼ��ʾ���̣��ù������Ʊ��� ���������衣
��ش��������⣺
��1�����չ�������Ŀ����___________��
��2���ڼ��������¼����ռ���Һʱ��������Ӧ�Ļ�ѧ����ʽΪ___________��__________��
��3�����������������ɳ��������ӷ���ʽΪ_________��
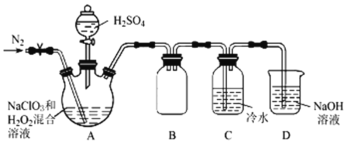
��4�������������������Ƴ�ˮ��Һ�������Һ�����е�����������ͼƬ�йص���________������ĸ����

���𰸡���ȥ�������е��л������� SiO2��2NaOH=Na2SiO3��H2O Al2O3��2NaOH=2NaAlO2��H2O SiO32-��2H��=H2SiO3�� AC
��������
��1���������е��л����ʿ���ͨ�����յķ�ʽ��ȥ��
��2�����������������������������������������߾��ܺ�ǿ�Ӧ��
��3����������Աȹ���ǿ����������м�������Ի�ù��
��4�����������������Ƴ�ˮ��Һ���γɵķ�ɢϵ�ǽ��壬����������Dz������������
(1)�������к�������Fe2O3��Al2O3���л�������ʣ���ȥ�л����ʿ��Բ������յķ�����
�ʴ�Ϊ����ȥ�������е��л������ʣ�
(2)������������������ܺ�ǿ�Ӧ,����ʽΪ��SiO2��2NaOH=Na2SiO3��H2O��Al2O3��2NaOH=2NaAlO2��H2O��
�ʴ�Ϊ��SiO2��2NaOH=Na2SiO3��H2O��Al2O3��2NaOH=2NaAlO2��H2O��
(3)��ѧ��Ӧ��ѭǿ��������Ĺ��ɣ�����H2SO4�ܺ�Na2SiO3��Ӧ�����ӷ���ʽΪ��SiO32-��2H��=H2SiO3����
�ʴ�Ϊ��SiO32-��2H��=H2SiO3����
(4)�����������������Ƴɵ�ˮ��Һʵ��Ϊ���壬����������в������������AͼΪ�����еĶ����ЧӦ��Bͼ��Cͼ�ֱ�Ϊһ����ͨ����Һ�ͽ���ʱ������
��ѡ��AC��

����Ŀ���ⶨ0.1 mol��L-1 Na2SO3��Һ�������ٽ��¹����е�pH���������¡�
ʱ�� | �� | �� | �� | �� |
�¶�/�� | 25 | 30 | 40 | 25 |
pH | 9.66 | 9.52 | 9.37 | 9.25 |
ʵ������У�ȡ�٢�ʱ�̵���Һ�����������ữ��BaCl2��Һ���Ա�ʵ�飬�ܲ�����ɫ�����ࡣ
����˵������ȷ����
A. Na2SO3��Һ�д���ˮ��ƽ����![]() +H2O
+H2O![]()
![]() +OH
+OH
B. ����pH������ͬ��������![]() Ũ�ȼ�С��ɵ�
Ũ�ȼ�С��ɵ�
C. �١����Ĺ��������¶Ⱥ�Ũ�ȶ�ˮ��ƽ���ƶ������Ӱ��һ��
D. ��������Kwֵ���