��Ŀ����
20�����;�ˮ���������K2FeO4Ϊ����ɫ���壬������ˮ�������Ի�������Һ���ֽ⣬�ڼ�����Һ���ȶ�����ҵ���Ʊ�K2FeO4�ij��÷��������֣�������������������������������ͼ��ʾ��
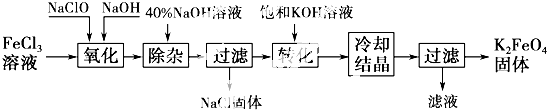
��1����ɡ������������з�Ӧ�Ļ�ѧ����ʽ��
FeCl3+10NaOH+3NaClO��2Na2FeO4+9NaCl+5H2O��������������NaClO���ѧʽ����
��2����ת���������з�����Ӧ�Ļ�ѧ����ʽΪNa2FeO4+2KOH=K2FeO4+2NaOH
��3���������յõ��ĸ�����س������� �ʣ������ؽᾧ���ᴿ�������ǣ����ֲ�Ʒ��ϡKOH��Һ�ܽ⣬Ȼ���ټ��뱥��KOH��Һ��ȴ�ᾧ�����ˣ�
������ⷨ������Ϊ�����������������Һ��Ȼ��������Һ�м���KOH��
��4�����ʱ����������Ӧ����FeO42-���õ缫��Ӧ����ʽΪFe+8OH--6e-=FeO42-+4H2O
��5����п�i������ƣ�K2FeOnҲ������ɼ��Ե�أ�K2FeO4�ڵ������Ϊ���� ���ϣ���缫��ӦʽΪFeO42-+3e��+4H2O=Fe��OH��3+5OH-���õ�ط�Ӧ�Ļ�ѧ����ʽΪ3Zn+2FeO42-+8H2O=3Zn��OH��2+2Fe��OH��3+4OH-��
��6��������Goethite�����Ե¹�ʫ�˸�£�Goethe�����������ģ����Ԫ����Fe��O��H����ѧʽ��Ϊ89����ѧʽ��FeO��OH����
���� ��1����Ӧ��NaClO������������ԭ������NaCl������Ԫ���غ㣬��֪��Ӧʽ����Ҫ����NaCl��H2O�����ݻ��ϼ���������ƽ���̣�
��2���ɹ������̿�֪�������������̳��Ӻ����Һ�к���Na2FeO4����ת�������̵IJ���ΪK2FeO4���ʡ�ת�����������ڼ���KOH��Һ��Na2FeO4ת��Ϊ�ܽ�ȸ�С��K2FeO4��
��3������Ŀ��Ϣ��֪��K2FeO4������ˮ�������Ի�������Һ���ֽܷ⣬�ڼ�����Һ���ȶ����ڷ������ᴿ��ʱ���Ҫ�ڼ��Ի����н��У�Ҫ��ֹ���������ʣ�������Ҫ��K2FeO4�ֲ�Ʒ��ϡKOH��Һ���ܽ⣬Ȼ����뱥��KOH��Һ����ȴ�ᾧ��
��4������Ŀ��Ϣ��֪����Ϊ�����������������Һ������FeO42-����Ԫ���غ㻹����ˮ��
��5��K2FeO4-ZnҲ������ɼ��Ե�أ�K2FeO4�ڵ������Ϊ�������ϣ�����Ϊпʧ���ӷ���������Ӧ���缫��ӦZn-2e-+2OH-=Zn��OH��2�����ݲ���͵����غ�д��������Ӧ��FeO42-+3e��+4H2O��Fe��OH��3+5OH������������Ӧ������Ӧ�ϲ��õ���ط�Ӧ��
��6�����ݻ�ѧʽ��Ϊ89�����Ԫ����Fe��O��H�������������ԭ����Ϊ56���۵�56ʣ��33����ѧʽ��ֻ�ܺ���2����ԭ�Ӻ�1����ԭ�ӣ����жϿ��ܵ���ɣ�
��� �⣺��1����Ӧ��FeCl3��Na2FeO4����Ԫ�ػ��ϼ���+3������Ϊ+6�ۣ����ϼ�������3�ۣ�NaClO��NaCl����Ԫ�ػ��ϼ���+1����Ϊ-1�ۣ����ϼ��ܹ�����2�ۣ����ϼ�������С������Ϊ6����FeCl3ϵ��Ϊ2��NaClOϵ��Ϊ3������Ԫ���غ��֪ Na2FeO4ϵ��Ϊ2������Ԫ���غ��֪NaClϵ��Ϊ2��3+3=9��������Ԫ���غ��֪NaOHϵ��Ϊ9+2��2=13������Ԫ���غ��֪H2Oϵ��Ϊ5����ƽ����ʽΪ2FeCl3+10NaOH+3NaClO=2Na2FeO4+9NaCl+5H2O��
��Ӧ��NaClO��NaCl����Ԫ�ػ��ϼ���+1����Ϊ-1�ۣ�NaClO������������ԭ������NaCl��
�ʴ�Ϊ��2��10��3��2��9NaCl��5H2O��NaClO��
��2����ת�����������ڼ���KOH��Һ��Na2FeO4ת��Ϊ�ܽ�ȸ�С��K2FeO4����Ӧ����ʽΪNa2FeO4+2KOH=K2FeO4+2NaOH��
�ʴ�Ϊ��Na2FeO4+2KOH=K2FeO4+2NaOH��
��3������Ŀ��Ϣ��֪��K2FeO4������ˮ�������Ի�������Һ���ֽܷ⣬�ڼ�����Һ���ȶ����ڷ������ᴿ��ʱ���Ҫ�ڼ��Ի����н��У�Ҫ��ֹ���������ʣ�������Ҫ��K2FeO4�ֲ�Ʒ��ϡKOH��Һ���ܽ⣬Ȼ����뱥��KOH��Һ����ȴ�ᾧ�����ˣ�
�ʴ�Ϊ��ϡKOH��Һ���ټ��뱥��KOH��Һ��ȴ�ᾧ�����ˣ�
��4������Ŀ��Ϣ��֪����Ϊ�����������������Һ������FeO42-�������缫��ӦʽΪFe+8OH--6e-=FeO42-+4H2O��
�ʴ�Ϊ��Fe+8OH--6e-=FeO42-+4H2O��
��5��ԭ��صĸ�������������Ӧ�������缫��ӦʽΪ����FeO42-+3e��+4H2O=Fe��OH��3+5OH-�������缫��ӦΪ����Zn-2e-+2OH-=Zn��OH��2�����ݵ缫��Ӧ�ĵ����غ㣬�١�2+�ڡ�3�ϲ��õ���ط�ӦΪ��3Zn+2FeO42-+8H2O=3Zn��OH��2+2Fe��OH��3+4OH-��
�ʴ�Ϊ��FeO42-+3e��+4H2O=Fe��OH��3+5OH-��3Zn+2FeO42-+8H2O=3Zn��OH��2+2Fe��OH��3+4OH-��
��6����ѧʽ��Ϊ89�����Ԫ����Fe��O��H�������������ԭ����Ϊ56���۵�56ʣ��33����ѧʽ��ֻ�ܺ���2����ԭ�Ӻ�1����ԭ�ӣ����Կ��ܵ����ΪFeO��OH����
�ʴ�Ϊ��FeO��OH����
���� ���⿼�鷽��ʽ����д��������ʵ��������Ķ���Ŀ��ȡ��Ϣ�����ȣ��Ѷ��еȣ���Ҫѧ���߱��ۺ�����֪ʶ����Ŀ��Ϣ�������⡢�����������������������Ŀ����Ҫ�����ÿһ����Ӧ�����������������ԭ����

��1��������Cu2O����Ϊ̫����ֽ�ˮ�Ĵ�����һ���¶��£���2L�ܱ������м�������Cu2O��ͨ��0.10molˮ����������Ӧ��2H2O��g��$?_{Cu_{2}O}^{����}$2H2��g��+O2��g����H=+484kJ•mol-1����ͬʱ�β���O2�������±���
| ʱ��/min | 20 | 40 | 60 | 80 |
| n��O2��/mol | 0.0010 | 0.0016 | 0.0020 | 0.0020 |
��2�����з�Ӧ��CO��g��+H2O��g��?CO2��g��+H2��g����H��0������ͬ�¶Ⱥ���ͬ����½��мס��ҡ�����������ʵ�飬ʵ����ʼʱ���������ڸ���ֵ����ʵ������±���
| ���ʵ��� | CO | H2 O | CO2 | H2 |
| �� | a mol | a mol | 0mol | 0mol |
| �� | 0mol | 0mol | 2a mol | a mol |
| �� | 0mol | 0mol | a mol | a mol |
| �� | a mol | a mol | a mol | a mol |
��3����������;�㷺����Ҫ��������Ӳ�ʻ����µĺϽ��Լ����ݵĵ�˿�������£����ܱ���������H2��ԭWO3�ý����٣��ܷ�ӦΪWO3 ��s��+3H2 ��g��$\stackrel{����}{?}$W ��s��+3H2O ��g������ش��������⣺
��ij�¶��·�Ӧ��ƽ��ʱ��H2��ˮ�����������Ϊ2��3����H2��ƽ��ת����Ϊ60%��
�������ܷ�Ӧ���̴��·�Ϊ�����Σ�������Ҫ�ɷ����¶ȵĹ�ϵ���±���ʾ��
| �¶� | 25�桫550�桫600�桫700�� |
| ��Ҫ�ɷ� | WO3 W2O5 WO2 W |
����˿�ƹ��е�W��ʹ�ù����л����ӷ���ʹ��˿��ϸ������I2���ӳ��ƹܵ�ʹ���������乤��ԭ��Ϊ��W��s��+2I2 ��g��$?_{3000��}^{1400��}$ WI4 ��g��������˵����ȷ����AB������ĸ����
A���ƹ��ڵ�I2��ѭ��ʹ��
B��WI4�ڵ�˿�Ϸֽ⣬������W�ֳ����ڵ�˿��
C��WI4�ڵƹܱ��Ϸֽ⣬ʹ�ƹܵ������ӳ�
D���¶�����ʱ��WI4�ķֽ����ʼӿ죬W��I2�Ļ������ʼ�����
| A�� | PH��7����ˮ��Ϊ���� | |
| B�� | ��������Ĵ����ŷ�����ɹ⻯ѧ��������Ҫԭ�� | |
| C�� | PM2.5��ָ������ֱ��С�ڻ����2.5�Ŀ������ΰ��ȼ����ķ������ | |
| D�� | SO2��CO2��NO2�����ڴ�����Ⱦ�� |


 ��
�� ��2��������þ��Mg2Si3O8?nH2O��������ˮ����ҽҩ�Ͽ���������������˿����к�θҺ�ж�����֮�⣬���ɵ�H2SiO3���ɸ������������θ���棬�����䲻���ܴ̼���������þ�����ᷴӦ�Ļ�ѧ����ʽΪMg2Si3O8?nH2O+4HCl�T2MgCl2+3H2SiO3+��n-1��H2O��
��2��������þ��Mg2Si3O8?nH2O��������ˮ����ҽҩ�Ͽ���������������˿����к�θҺ�ж�����֮�⣬���ɵ�H2SiO3���ɸ������������θ���棬�����䲻���ܴ̼���������þ�����ᷴӦ�Ļ�ѧ����ʽΪMg2Si3O8?nH2O+4HCl�T2MgCl2+3H2SiO3+��n-1��H2O��