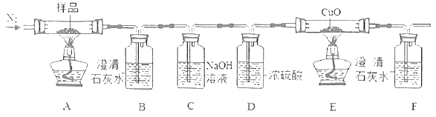
��Ŀ����
����Ŀ�������й�˵����ȷ�ҽ��ͺ�������
˵�� | ���� | |
A | һ���¶�ѹǿ�£�2 g H2 �� 4 g H2 ��ȫȼ�գ����� ȼ���ȵ���ֵ�� | 4 g H2 �ų������� |
B | 2SO2(g)��O2(g) ƽ����ټ��� SO2��Q ���� | ƽ�����ƣ��ų��������� |
C | ������ʵ���Ũ�ȵ� NaI �� KBr ���Һ�еμ� AgNO3 ��Һ�������ɻ�ɫ AgI ���� | Ksp(AgI)��Ksp(AgBr) |
D | �����ʵ���Ũ�� Na2CO3 �� pH ���� CH3COONa | H2CO3 �����Ա� CH3COOH ǿ |
A.AB.BC.CD.D
���𰸡�C
��������
A. ������������Խ��ȼ�շų���������Խ�࣬4 g H2 �ų������ࣻ��ȼ����ָ����1mol��ȼ����ȫȼ�������ȶ���������ʱ�ų���������һ���¶�ѹǿ�£�������ȼ���ȵ���ֵ�Ǹ���ֵ��A������
B. ��һ����Ӧȷ���Ժ����÷�Ӧ�ġ�H�Ͳ��ٷ����仯��������Ӧ��ƽ����ټ��� SO2��ƽ�����ƣ��ų��������������ǡ�H������B������
C. ������ʵ���Ũ�ȵ� NaI �� KBr���Һ�еμ�AgNO3 ��Һ�������ɻ�ɫ AgI ������˵�������Ӻ��������ȷ�����Ӧ���ɸ����ܵij�����Ksp(AgI)��С������Ksp(AgI)��Ksp(AgBr)��C��ȷ��
D. �����ʵ���Ũ�� Na2CO3 �� pH ���� CH3COONa��˵��Na2CO3��ˮ����������CH3COONa��ˮ������������Խ��Խˮ��Ĺ��ɿ�֪���γɸ��ε���Խ������ˮ��������Խǿ����������H2CO3 �����Ա� CH3COOH ����D������
��������������ѡC��

����Ŀ���±���Ԫ�����ڱ���һ���֣��������е���ĸ�ֱ����һ��Ԫ�ء�
A | |||||||||||||||||
B | C | D | E | F | T | ||||||||||||
G | H | I | J | K | L | ||||||||||||
M | N | O | |||||||||||||||
�Իش��������⣨ע�⣺ÿ���е���ĸ����Ϊ�ϱ��е���ĸ���ţ�����ΪԪ�ط��ţ�
(1)N�ĵ��ʺ�ˮ������Ӧ�����ɹ���X����I�ĵ�����X��Ӧ�Ļ�ѧ����ʽ_______��
(2)D����̬�⻯���VSEPRģ�͵�����Ϊ_______��
(3)��A��C��D�γɵ�ACD�����У�����������������= _______________��
(4)Ҫ֤��̫�����Ƿ���R Ԫ�أ��ɲ��õķ�����__________________________��
(5)Ԫ��M�Ļ�����(ME2L2)���л��ϳ��п������������Ȼ��������������л��ﷴӦ���ش����⣺
��ME2L2������Ϊ���ɫҺ�壬����CCl4��CS2�Ȼ��ܣ��ݴ˿��ж�ME2L2��_________������ԡ��Ǽ��ԡ������ӡ�
�ڽ�N��O�ĵ����õ������Ӻ����D������������Ӧ��ˮ����Ũ��Һ�У����Ƴ�ԭ��أ�����ɸ������ϵ�Ԫ�ص���Χ���ӹ����ʾʽΪ______________________��
(6)��O2����Һ�м��백ˮ���γ���ɫ�������������백ˮ���������ܽ�����ɫ����Һ��д�������ܽ�����ӷ���ʽ_____��
(7)��F ��K����Ԫ���γɵĻ�����������ԭ�ӵļ۵���ȫ������ɼ�����û�����Ŀռ乹�͵�����Ϊ___��
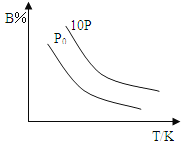
(8)��ͼ�������߷ֱ��ʾ��A�塢��A�塢��A�塢��A��Ԫ����̬�⻯��е�仯����E���⻯�����ڵ�������__����m��n��x��y����
(9)1183 K���´�N����Ļ����ṹ��Ԫ��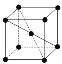 ��ʾ��1183 K����ת��Ϊ
��ʾ��1183 K����ת��Ϊ ��ʾ�ṹ�Ļ����ṹ��Ԫ����1183 K���µľ����У��ռ�������Ϊ____����1183 K���ϵľ����У���Nԭ�ӵȾ����������Nԭ����Ϊ____������ѻ���ʽ������Ϊ_____��
��ʾ�ṹ�Ļ����ṹ��Ԫ����1183 K���µľ����У��ռ�������Ϊ____����1183 K���ϵľ����У���Nԭ�ӵȾ����������Nԭ����Ϊ____������ѻ���ʽ������Ϊ_____��
����Ŀ��CCuS��һ�ֶ�����̼�IJ�����������ļ��������ּ����ɽ�CO2��Դ���� ��������Ч�档CO2��������������ɵ�̼�л����Ҫ�����·�Ӧ��
��ӦI��CO2(g)��3H2(g) ![]() CH3OH(g)��H2O(g) ��H1=��49.8kJ��mol��1
CH3OH(g)��H2O(g) ��H1=��49.8kJ��mol��1
��ӦII��CH3OCH3(g)��H2O(g) ![]() 2CH3OH(g) ��H2=��23.4kJ��mol��1
2CH3OH(g) ��H2=��23.4kJ��mol��1
��ӦIII��2CO2(g)��6H2(g) ![]() CH3OCH3(g)��3H2O(g) ��H3
CH3OCH3(g)��3H2O(g) ��H3
��1����H3=_____________kJ��mol��1
��2�����º��������£����ܱ�������ͨ�˵����ʵ�����CO2��H2��������ӦI������������˵����ӦI�ﵽƽ��״̬����_______(����ţ���
A.�����ڵĻ��������ܶȱ��ֲ���B.��Ӧ��ϵ��ѹǿ���ֲ���
C.CH3OH��CO2��Ũ��֮�ȱ��ֲ���D.����3NA��H-O��ͬʱ����2NA��C=O��
��3����ӦII��ij�¶��µ�ƽ�ⳣ��Ϊ0.25,���¶��£����ܱ������м��˵����ʵ����� CH3OCH3(g)��H2O(g)����Ӧ��ijʱ�̲�ø����Ũ�����£�
���� | CH3OCH3(g) | H2O(g) | CH3OH(g) |
Ũ��/mol��L��1 | 1.6 | 1.6 | 0.8 |
��ʱ![]() ___
___![]() ������>������<������=����������Ӧ�ﵽƽ��״̬ʱ�����������CH3OH ������� V(CH3OH)%= _____%��
������>������<������=����������Ӧ�ﵽƽ��״̬ʱ�����������CH3OH ������� V(CH3OH)%= _____%��
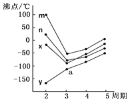
��4����ijѹǿ�£���ӦIII�ڲ�ͬ�¶ȡ���ͬͶ�ϱ�ʱ��CO2��ƽ��ת������ͼ��ʾ��T1�¶��£���6 mol CO2��12 mol H2����3 L���ܱ������У�10 min��Ӧ�ﵽƽ��״̬����0-10 min�ڵ�ƽ����Ӧ����V(CH3OCH3)=____��
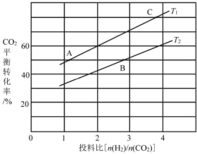
��5����ѹ�½�CO2���ϰ������1 ��3��ϣ��� ��ͬ���������·�����ӦI�ͷ�ӦIII������ͬ��ʱ�����CH3OH��ѡ���ԺͲ������¶ȵı仯����ͼ��
���У�CH3OH��ѡ����=![]() ��100%
��100%
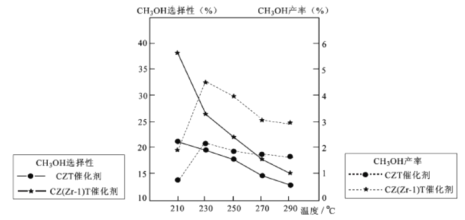
���¶ȸ���230����CH3OH�������¶����߶��½���ԭ����________��
�������������ºϳɼ״��Ĺ�ҵ������_________��
A. 230�� B. 210�� C.���� CZT D.���� CZ(Zr-1)T