��Ŀ����
2��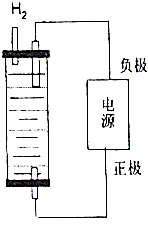 ��ȡƯ�۵�����ͨ����ⱥ��ʳ��ˮ�õ���
��ȡƯ�۵�����ͨ����ⱥ��ʳ��ˮ�õ�����1��������ȡƯ��2.54�֣���������Ҫ��״̬�����������Ϊ���٣���Ҫ����NaCl�������Ƕ��٣���д��������̣�
��2��ij�о���ѧϰС������ͼ���װ�õ��500g����ʳ��ˮһ��ʱ�䣮�������з���ֻ�������ų����о�������Һ������Һ��ǿƯ���ԣ���Ư�����ʿ���ΪNaClO����ʱ��·�й�ͨ��4mol���ӣ����㱾��ʵ��������Һ�и�Ư�������ʵ���������56%��
���� ��1��Ư�۵�Ϊ������ƺ��Ȼ��ƵĻ���2.54�ֵĴ�����ƺ��Ȼ��Ƶ����ʵ���Ϊ��$\frac{2.54��1{0}^{6}g}{254g/mol}$=104mol����Ϸ���ʽ���㼴�ɣ�
��2����ⱥ��ʳ��ˮ����������ʧ���ӷ���������Ӧ�������������������ӵõ����ӷ�����ԭ��Ӧ�����������������������������ɴ������ƣ��ݴ˽�ɣ�
��� �⣺��1������Ҫ���������ʵ���Ϊxmol��
Ư�۵���ȡԭ��Ϊ��2Cl2+2Ca��OH��2�TCa��ClO��2+CaCl2+2H2O��
2mol 143g+111g
xmol 2.54��106g
��x=2��104mol������Ҫ��������Ϊ��22.4L/mol��2��104mol=4.48��105L��
����Ҫ�Ȼ��Ƶ�����Ϊyg��
2NaCl+2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2NaOH+Cl2��+H2��
2��58.5g 1mol
yg 2��104mol
��y=2.34��106g������������Ҫ��״̬�����������Ϊ4.48��105L����Ҫ����NaCl��������2.34��106g��
��2����ⱥ��ʳ��ˮ����������ʧ���ӷ���������Ӧ�������������������ӵõ����ӷ�����ԭ��Ӧ������������Ӧ�Ļ�ѧ����ʽΪ2NaCl+2H2O$\frac{\underline{\;���\;}}{\;}$H2��+Cl2��+2NaOH�������������������������ɵĴ������ƾ���Ư���ԣ���ѧ��Ӧ����ʽΪ��Cl2+2NaOH=NaCl+NaClO+H2O����ÿת��2mol��������1mol������ÿ��1mol���������գ������ɴ�������Ϊ1mol����ôNaClO����������Ϊ��$\frac{74.5}{133}$��100%=56%���ʴ�Ϊ��NaClO��56%��
���� ������Ҫ������ǵ��ʳ��ˮ��ԭ�����漰��ѧ��Ӧ����ʽ���й����ʵ����������ļ��㣬�ѶȲ���

 �¿α�ͬ��ѵ��ϵ�д�
�¿α�ͬ��ѵ��ϵ�д� һ����ʦ����Ӧ����������һ��ȫϵ�д�
һ����ʦ����Ӧ����������һ��ȫϵ�д� Сѧѧϰ�ð���ϵ�д�
Сѧѧϰ�ð���ϵ�д�| A�� |  �ȽϷǽ�����ǿ��Cl��C��Si | B�� |  �Ʊ��������� | ||
| C�� |  ʵ���������� | D�� |  ʵ�����ƶ�����̼ |
| A�� | ������Һ�м���CuSO4��Һ�������ʷ������� | |
| B�� | һ�������£��Ҵ��ܱ�������һ�ִ̼�����ζ��Һ�� | |
| C�� | ��Cl2��NaOH��ҺΪԭ���Ʊ��������� | |
| D�� | ��ˮ������������������о��漰������ԭ��Ӧ |
| A�� | 1��3 | B�� | 1��1 | C�� | 2��1 | D�� | 3��1 |
| A�� | ��һ�����Ĵ�����һ������NaOH��Һ��ϣ�����Ϻ���Һ�д��ڣ�c��CH3COO-��+c��CH3COOH��=2c��Na+����ʽ����Ҳһ������c��OH-��+c��CH3COO-��=c��CH3COOH��+c��H+�� | |
| B�� | ��Ũ�ȡ��������Na2CO3��Һ��NaHCO3��Һ��ϣ�$\frac{c��HC{{O}_{3}}^{-}��}{c��{H}_{2}C{O}_{3}��}��\frac{c��C{{O}_{3}}^{2-}��}{c��HC{{O}_{3}}^{-}��}$ | |
| C�� | �����£�V1 L pH=11����Һ��V2 L pH=3��HA��Һ��Ϻ������ԣ���V1��V2 | |
| D�� | ��֪A-+H2B��������=HA+HB-��������ǿ��˳��һ��Ϊ��HB-��HA��H2B |
| A�� | 2.0gH218O��D2O�Ļ����������������ΪNA | |
| B�� | ���³�ѹ�£�4.4 g��ȩ�����Ҽ���ĿΪ0.7NA | |
| C�� | ��״���£�5.6LCO2������Na2O2��Ӧת�Ƶĵ�����Ϊ0.5NA | |
| D�� | 50mL12mol/L����������MnO2���ȣ�ת�Ƶĵ�����Ϊ0.3NA |
| A�� | �����ʵ�����ˮ����ˮ���е������� | |
| B�� | ����������ϩ�ͱ�ϩ�к��еĹ��е��Ӷ��� | |
| C�� | ͬ�¡�ͬѹ��ͬ�����CO��NO���е������� | |
| D�� | �����ʵ������������ֱ�������������ȫ��Ӧʱת�Ƶĵ����� |

�ٰ���ͼ���Ӻ�װ�ã����װ�������ԣ�
�ڳ�ȡ���� Fe2O3��ʯӢ�Թ��У���ȼ�ƾ��ƣ�����������ᣮ
�������ij�������ȼ���������ƾ��ƣ�
��30min��Ϩ��ƾ��ƣ��رյ��ɼУ�
�ݴ�������ȴ�����º��ռ����
�������Ϸ����ֱ��ռ����������־ƾ��ƣ��������ֿ��Լ��л��桢����¶ȣ��;ƾ���Ƽ��ȵIJ��
��ش��������⣺
��1���Ʊ�CO��ԭ�������ü��ᣨHCOOH����Ũ������������µķֽ��Ƶã�ʢ�ż������������Ϊ��Һ©�����÷�Ӧ�Ļ�ѧ����ʽHCOOH $��_{��}^{Ũ����}$CO��+H2O��
��2��ʵ�鲽���ij�������ָ����CO���ȣ�
��3��ʵ�鲽���Ϩ��ƾ��Ƶ�˳��Ϊ��I��II�������
��4��ͨ�������ϻ�ȡ������Ϣ��
�ƾ���ƽ���¶�Ϊ600�棻�����־ƾ���ƽ���¶�Ϊ700�棬�ƾ����ƽ���¶�Ϊ930�森
������ָ������Ӧ�¶ȸ���710�棬Fe���ȶ����ڣ�680�桫710��֮�䣬FeO�ȶ����ڣ�����680�棬����Ҫ��Fe3O4���Է����ƾ��Ƽ�������������Fe��ԭ���dz�ʱ�伯�м���ʹ�ֲ��¶ȴﵽ��ԭ����������Ҫ���¶ȣ�
��5����֪FeO��Fe2O3��Fe3O4��Ԫ�ص����������ֱ�Ϊ��22.2%��30%��27.6%��
���������������3����Ʒ����Ԫ�������Ԫ�ص������������±���
| ���ȷ�ʽ | ����Ԫ����� | ��Ԫ�ص���������% | |
| Fe | O | ||
| �ƾ��� | Fe��O | 74.50 | 25.50 |
| �����־ƾ��� | Fe��O | 76.48 | 23.52 |
| �ƾ���� | Fe | 100.00 | 0.00 |
��6��ͨ����һ���������������ǰ���ּ��ȷ�ʽ�õ��Ĺ����ĩ�ɷ־�ΪFe3O4��Fe���þƾ���Ƽ��ȵõ��Ĺ����ĩ�ɷ�ΪFe����������þƾ��Ƽ��ȷ�ʽ�������Fe3O4��Fe��������Ϊ12��1����Ҫ����������