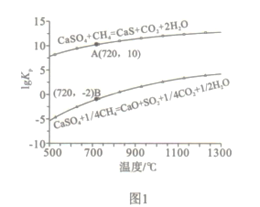
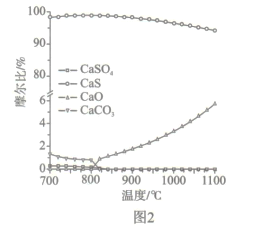
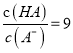��Ŀ����
����Ŀ��������ѧ֪ʶ��д���пհס�
��1����֪��1mol H��H����1mol N��H����1mol N��N���ֱ���Ҫ��������436kJ��391kJ��946kJ����N2��H2��Ӧ����1mol NH3�ķ�Ӧ�Ȧ�H =__________________��
��2��������ȼ����Ϊ286kJ/mol��д����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽ____________________________________________��
��3��ij�¶��´�ˮ�е�c(H+) = 1��10��6.5mol/L�����¶Ȳ��䣬����ϡ����ʹc(H+)= 5��10��5mol/L������ˮ�������c(H+) =__________mol/L��
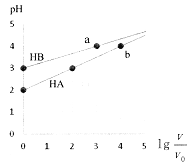
��4����֪�ڵڣ�3���������£������pH=a������������pH=b�����������1:10��Ϻ���Һ�����ԣ���a+b=_________��
��5��ij��Ԫ�ᣨ��ѧʽ��H2B��ʾ����ˮ�еĵ��뷽��ʽ��H2B=H��+HB��, HB�� ![]() H��+B2����
H��+B2����
��֪0.1 mol/L NaHB��Һ��pH=2����0.1 mol/L H2B��Һ��c(H+)С��0.11 mol/L��ԭ����____________________________________��
���𰸡� ��H����46kJ/mol H2(g)+1/2O2(g)=H2O ��H����286kJ/mol 2��10��9 12 H2B��һ�����������H��������HB���ĵ���
��������(1)��Ӧ�ȵ��ʱ���H=��Ӧ���ܼ���-�������ܼ�����N2��H2��Ӧ����NH3�ķ�Ӧ����ʽΪN2 + 3H2 ![]() 2NH3������H =946kJ/mol+436kJ/mol��3-391kJ/mol��6=��46kJ/mol���ʴ�Ϊ����46kJ/mol��
2NH3������H =946kJ/mol+436kJ/mol��3-391kJ/mol��6=��46kJ/mol���ʴ�Ϊ����46kJ/mol��
(2)������ȼ����Ϊ286kJ/mol��������ȼ���ȵ��Ȼ�ѧ����ʽΪH2(g)+1/2O2(g)=H2O ��H����286kJ/mol���ʴ�Ϊ��H2(g)+1/2O2(g)=H2O ��H����286kJ/mol��
(3)ij�¶��´�ˮ�е�c(H+) = 1��10��6.5mol/L����Kw=1��10��6.5mol/L��1��10��6.5mol/L=1��10��13�����¶Ȳ��䣬����ϡ����ʹc(H+)= 5��10��5mol/L����c(OH-)=![]() mol/L =2��10��9 mol/L�������ˮ�������c(H+) =2��10��9mol/L���ʴ�Ϊ��2��10��9��
mol/L =2��10��9 mol/L�������ˮ�������c(H+) =2��10��9mol/L���ʴ�Ϊ��2��10��9��
(4)��֪�ڵ�(3)�������£������pH=a��c(H+) =1��10��amol/L������������pH=b��c(H+) =1��10��bmol/L����c(OH-)=1��10��(13-b)mol/L�����������1:10��Ϻ���Һ�����ԣ�˵����������������ǡ����ȫ��Ӧ�����1��10��amol/L��V=1��10��(13-b)mol/L��10V�����a+b=12���ʴ�Ϊ��12��
(5)0.1 mol/L NaHB��Һ��pH=2����c(H+) =0.01mol/L����0.1 mol/L H2B��Һ�д���H2B=H��+HB��, HB�� ![]() H��+B2����H2B��һ�����������H��������HB���ĵ��룬����0.1 mol/L H2B��Һ��c(H+) ��0.11mol/L���ʴ�Ϊ��H2B��һ�����������H��������HB���ĵ�����
H��+B2����H2B��һ�����������H��������HB���ĵ��룬����0.1 mol/L H2B��Һ��c(H+) ��0.11mol/L���ʴ�Ϊ��H2B��һ�����������H��������HB���ĵ�����

 ����ѧ���ʱѧ����ϵ�д�
����ѧ���ʱѧ����ϵ�д� �ʰ�Ӣ��ͬ����ϰ��ϵ�д�
�ʰ�Ӣ��ͬ����ϰ��ϵ�д�