��Ŀ����
����Ŀ���±��������dz�����HClO��H2CO3�ĵ��볣�����ش��й����⡣
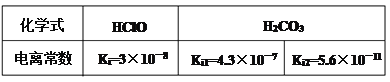
��1����������ȷ��ʾˮ������ӷ���ʽ����______________��
A��HCO3�� + H2O![]() H2CO3+OH�� B��HClO + H2O
H2CO3+OH�� B��HClO + H2O![]() ClO��+ H3O+
ClO��+ H3O+
C��HCO3�� + H2O![]() CO32��-+H3O+ D��CO32��+2H2O
CO32��-+H3O+ D��CO32��+2H2O ![]() H2CO3+2OH��
H2CO3+2OH��
��2��Ũ����ͬ�Ģ�Na2CO3����NaHCO3����NaClO������Һ��pH��С�������е�˳��Ϊ(�ñ����д)________________________��
��3��д��NaClO��Һ��ͨ������CO2��������ӷ���ʽ________________________��
��4����������ˮ�м�������̼�����Ʒ�ĩ����ˮ��Ư��������ǿ�������йػ�ѧ֪ʶ����ԭ��_____________________________________________��
��5���ڵ�Ũ�ȵ�NaClO��NaHCO3�����Һ�У�������Ũ�ȵĴ�С��ϵ��__________��
��6����pH=8��NaClO��HClO���Һ�У�c(ClO��)/c(HClO)=____________________��
��7����֪HClO��aq����NaOH��aq����Ӧ�Ħ�H =��a kJ/mol��HCl��aq����Һ��NaOH��aq����Ӧ�Ħ�H =��b kJ/mol����HClO��ˮ��Һ�е���Ħ�H����____________��
��8��pH=12��NaClO��Һ�У�c(HClO)=___________mol/L������ȷ���
���𰸡� A ��<��<�� ClO��+CO2+H2O��HClO+HCO3�� ������ˮ�д��ڿ��淴ӦCl2��H2O![]() HCl��HClO������NaHCO3��NaHCO3��HCl��ӦʹHClŨ�ȱ�С��ƽ�����ƣ�NaHCO3����HClO��Ӧ������HClOŨ��������ˮƯ��������ǿ c(HCO3��)>c(ClO��)>c(OH��)>c(CO32��) 3 +��b-a��kJ/mol 10-2-10-12
HCl��HClO������NaHCO3��NaHCO3��HCl��ӦʹHClŨ�ȱ�С��ƽ�����ƣ�NaHCO3����HClO��Ӧ������HClOŨ��������ˮƯ��������ǿ c(HCO3��)>c(ClO��)>c(OH��)>c(CO32��) 3 +��b-a��kJ/mol 10-2-10-12
��������(1)A��HCO3�� + H2O![]() H2CO3+OH����ʾ̼��������ӵ�ˮ�⣬��ȷ��B��HClO + H2O
H2CO3+OH����ʾ̼��������ӵ�ˮ�⣬��ȷ��B��HClO + H2O![]() ClO��+ H3O+�Ǵ�����ĵ��룬����C��HCO3�� + H2O
ClO��+ H3O+�Ǵ�����ĵ��룬����C��HCO3�� + H2O![]() CO32��-+H3O+��̼��������ӵĵ��룬����D��CO32��ˮ��ֲ����У�����ѡA��
CO32��-+H3O+��̼��������ӵĵ��룬����D��CO32��ˮ��ֲ����У�����ѡA��
(2)���ݱ������ݣ����ԣ�̼������̼��������ӣ����Ũ����ͬ�Ģ�Na2CO3����NaHCO3����NaClO������Һ��ˮ��̶Ȣ��������ڣ�ˮ��̶�Խ����Һ�ļ���Խǿ��pHԽ��pH��С�������е�˳��Ϊ���ʴ�Ϊ����<��<����
(3)���ԣ�̼������̼��������ӣ���NaClO��Һ��ͨ������CO2���巴Ӧ���ɴ������̼�����ƣ���Ӧ�����ӷ���ʽΪClO��+CO2+H2O��HClO+HCO3�����ʴ�Ϊ��ClO��+CO2+H2O��HClO+HCO3����
(4)������ˮ�д��ڿ��淴ӦCl2��H2O![]() HCl��HClO������NaHCO3��NaHCO3��HCl��ӦʹHClŨ�ȱ�С��ƽ�����ƣ�NaHCO3����HClO��Ӧ������HClOŨ��������ˮƯ��������ǿ���ʴ�Ϊ��������ˮ�д��ڿ��淴ӦCl2��H2O
HCl��HClO������NaHCO3��NaHCO3��HCl��ӦʹHClŨ�ȱ�С��ƽ�����ƣ�NaHCO3����HClO��Ӧ������HClOŨ��������ˮƯ��������ǿ���ʴ�Ϊ��������ˮ�д��ڿ��淴ӦCl2��H2O![]() HCl��HClO������NaHCO3��NaHCO3��HCl��ӦʹHClŨ�ȱ�С��ƽ�����ƣ�NaHCO3����HClO��Ӧ������HClOŨ��������ˮƯ��������ǿ��
HCl��HClO������NaHCO3��NaHCO3��HCl��ӦʹHClŨ�ȱ�С��ƽ�����ƣ�NaHCO3����HClO��Ӧ������HClOŨ��������ˮƯ��������ǿ��
(5)�ڵ�Ũ�ȵ�NaClO��NaHCO3�����Һ�д���������ӵ�ˮ��̶ȴ���c(HCO3��)>c(ClO��)��̼��������ӵ�ˮ��̶ȴ��ڵ���̶ȣ���Һ�е�������������2����ˮ�����ɣ����̼������ӵ�Ũ����С��������Ũ�ȵĴ�С��ϵΪc(HCO3��)>c(ClO��)>c(OH��)>c(CO32��)���ʴ�Ϊ��c(HCO3��)>c(ClO��)>c(OH��)>c(CO32��)��
(6)��pH=8��NaClO��HClO���Һ�У�![]() =
=![]() =
=![]() =
=![]() =3���ʴ�Ϊ��3��
=3���ʴ�Ϊ��3��
(7)��HClO(aq)H+(aq)+ClO-(aq)��H1����H+(aq)+OH-(aq)=H2O(l)��H2=-bkJmol-1����OH-(aq)+HClO(aq)=CN-(aq)+H2O(l)����H3=-akJmol-1�����ݸ�˹���ɣ���+��=������H1+��H2=��H3����H1=+(b-a)kJmol-1���ʴ�Ϊ��+(b-a)kJmol-1��
(8)pH=12��NaClO��Һ�д��������غ�c(Na+)= c(ClO��)+c(HClO)������غ�c(Na+)+ c(H+)= c(ClO��)+ c(OH-)����c(HClO)= c(H+)- c(OH-)=10-2-10-12 mol/L���ʴ�Ϊ��10-2-10-12��

 Сѧѧϰ�ð���ϵ�д�
Сѧѧϰ�ð���ϵ�д� Сѧͬ�����������ܾ�ϵ�д�
Сѧͬ�����������ܾ�ϵ�д�����Ŀ��ij���������������������� Fe2O3�� Fe3O4�� Al2O3�� CaO�� SiO2 �ȣ�Ϊԭ����ȡ������ Fe2O3��Ҫ��>99.2%�� CaO ����<0.01%�����乤����������(�������Լ����Թ���)��

��֪��������������� pH ���±���ʾ
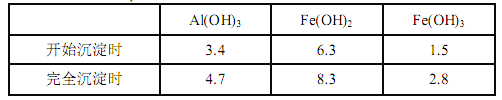
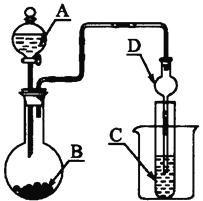
��1������ A ����Ҫ�ɷ���__________��
��2���ڹ��̢��пɹ۲쵽�����������ݣ���Һ��ɫ������dz���ܽ���ʵ����������ӷ���ʽ��__________��
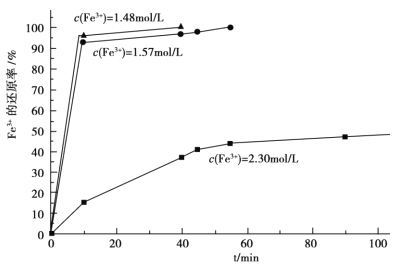
��3���ڹ��̢��У����������Һ A ϡ�Ͳ�ͬ������,����������Ĺ�������,�ó� Fe3+Ũ�ȡ���ԭ�ʺͷ�Ӧʱ��Ĺ�ϵ��ͼ��ʾ���������ʵ����˵����������ѡ��ϡ�ͺ�c(Fe3+)Ϊ 1.60mol/L ���ҵ�������______��
��4���ڹ��̢��У�����������ͬ�����£���ѡ���˲�ͬ���Ƽ�����ʵ�飬ʵ�����ݼ��±�������֪����Һ B �иƵĺ����� CaO ��Ϊ 290��310mg/L��
���Ƽ� | Na2SO3 | H2C2O4 | (NH4)2CO3 | Na2CO3 | NH4F |
����/g | 2 | 2 | 2 | 5 | 2 |
ʣ��CaO/mg/L) | 290 | 297 | 290 | 190 | 42 |
����ʵ������ ѡ�����˵ij��Ƽ����õ����� C ����Ҫ�ɷ���__________��
��5���ڹ��̢��У���Ӧ�¶���Ҫ������ 35�����£����˹��ߣ�����ܵ�ԭ����__________��
��6���ڹ��̢��У���Ӧ�Ļ�ѧ����ʽ��__________��
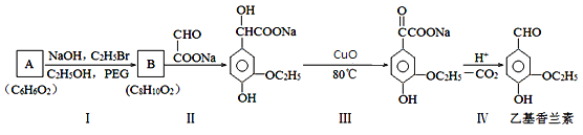
����Ŀ����þ������þ��������ɰ����(Na2B4O7��10H2O)ʱ�ķ���������Ҫ�ɷ���MgO��������Na2B4O7��CaO��Fe2O3��FeO��MnO��SiO2�����ʡ�����þ��Ϊԭ����ȡ��ˮ����þ�Ĺ����������£�

�ش��������⣺
(l)Na2B4O7��10H2O��B�Ļ��ϼ�Ϊ__________��
(2)Na2B4O7������ˮ��Ҳ����ˮ�⣺B4O72-+7H2O![]() 4H3BO3(����)+2OH-(�����ڳ������ܽ�Ƚ�С)��д����������ʱNa2B4O7������Ӧ�Ļ�ѧ����ʽ��______________��
4H3BO3(����)+2OH-(�����ڳ������ܽ�Ƚ�С)��д����������ʱNa2B4O7������Ӧ�Ļ�ѧ����ʽ��______________��
(3)����B�к��в�����ϡ���ᵫ��������Ũ����ĺ�ɫ���壬д�����ɺ�ɫ��������ӷ���ʽ____________��
(4)����MgO��Ŀ����___________________��
(5)��֪MgSO4��CaSO4���ܽ�����±���
�¶�(��) �ܽ��(g) | 40 | 50 | 60 | 70 |
MgSO4 | 30.9 | 33.4 | 35.6 | 36.9 |
CaSO4 | 0.210 | 0.207 | 0.201 | 0.193 |
������A���ǽ�MgSO4��CaSO4�����Һ�е�CaSO4��ȥ�������ϱ����ݣ���Ҫ˵��������A������Ϊ____________________��
(6)��ɰҲ���ڹ�ҵ����ȡNaBH4��NaBH4����Ϊ�л���ѧ�еġ����ܻ�ԭ������
��д��NaBH4�ĵ���ʽ��___________��
�ڡ���Ч�⺬�����������������ԭ���Ļ�ԭ�������䶨���ǣ�ÿ�˺��ԭ���Ļ�ԭ�����൱�ڶ��ٿ�H2�Ļ�ԭ������NaBH4����Ч�⺬��Ϊ_________��������λС������
���ڼ��������£��������ϵ��NaBO2Ҳ���Ƶ����⻯�ƣ�д�������ҵĵ缫��Ӧʽ��________��