��Ŀ����
ij����֣���Ҫ�ɷ�ΪFe����Ʒ�к�������ͭ����ȣ������ɷֺ��ԣ���Ϊ�˲ⶨ�úϽ������ĺ���������������¹������̣�
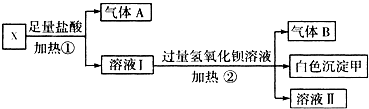
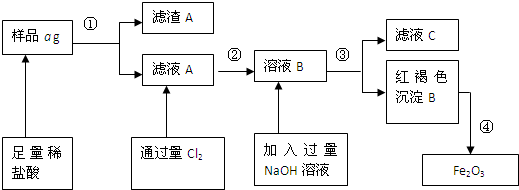
��Fe2O3����ҺB�������NaOH��Һ���ɫ����B�ڢ���Ʒag����A��ҺA����ϡ����ͨ����Cl2��ҺC
��1��ȡ��Ʒag����ȡʱʹ�õ���Ҫ��������Ϊ______��
��2����ҺA��������Ҫ����______��______��д��ѧʽ���������ٵ�����______����ʹ�õIJ���������______��
��3��д������ҺB���ɺ��ɫ����B�����ӷ���ʽ______��
��4��������Fe2O3�������������Ϊbg���������Ʒ����Ԫ�ص������ٷ����ı���ʽΪ���ú�a��b��ʽ�ӱ�ʾ��______��
��5�������֤��ҺA�к�Fe2+��������Fe3+______��
��Fe2O3����ҺB�������NaOH��Һ���ɫ����B�ڢ���Ʒag����A��ҺA����ϡ����ͨ����Cl2��ҺC

��1��ȡ��Ʒag����ȡʱʹ�õ���Ҫ��������Ϊ______��
��2����ҺA��������Ҫ����______��______��д��ѧʽ���������ٵ�����______����ʹ�õIJ���������______��
��3��д������ҺB���ɺ��ɫ����B�����ӷ���ʽ______��
��4��������Fe2O3�������������Ϊbg���������Ʒ����Ԫ�ص������ٷ����ı���ʽΪ���ú�a��b��ʽ�ӱ�ʾ��______��
��5�������֤��ҺA�к�Fe2+��������Fe3+______��
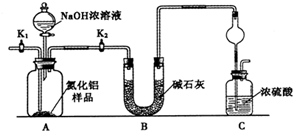
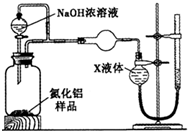
����֣���Ҫ�ɷ�ΪFe����Ʒ�к�������ͭ�ȣ���������ϡ�����������ܽ�Ϊ�Ȼ��������Ȼ�����Һ��ͭ���ܽ⣬���˵õ�����AΪͭ����ҺA�к��е����ʳɷ�ΪFeCl2��AlCl3��ͨ���������Ȼ���������Ϊ�Ȼ�����2FeCl2+Cl2=2FeCl3���õ���ҺB��ΪFeCl3��AlCl3��B��Һ�м����������������Һ��ӦΪ��FeCl3+3NaOH=Fe��OH��3��+3NaCl��AlCl3+4NaOH=NaAlO2+3NaCl�����˵õ����ɫ����������������ҺC����ҺC�к��е�����NaAlO2��NaCl��NaOH�����ɫ���������������ȷֽ�õ���������
��1��ȡ��Ʒag����ȡʱʹ�õ���Ҫ��������Ϊ������ƽ���ʴ�Ϊ��������ƽ��
��2����������ϡ�����������ܽ�Ϊ�Ȼ��������Ȼ�����Һ��ͭ���ܽ⣬���˵õ�����AΪͭ����ҺA�к��е����ʳɷ�ΪFeCl2��AlCl3�������ܽ��ͨ�������ٷ������Һ�������������������ǹ��ˣ���Ҫ�õ��IJ�������Ϊ��©�������������ձ���
�ʴ�Ϊ��FeCl2��AlCl3�����ˣ�©�������������ձ���
��3����ҺA����FeCl2��AlCl3�������������������Һ�õ����ɫ����������������Һƫ��������Һ����ҺB���ɺ��ɫ����B�����ӷ���ʽΪ��Fe3++3OH-=Fe��OH��3�����ʴ�Ϊ��Fe3++3OH-=Fe��OH��3����
��4����Ʒ����Ϊag������Fe2O3�������������Ϊbg��������Ԫ���غ�������Ʒ����Ԫ����������=
��100%=
��100%��
�ʴ�Ϊ��
��100%��
��5����ҺA�к��е����ʳɷ�ΪFeCl2��AlCl3����֤��ҺA�к�Fe2+��������Fe3+�����������Ӻ��������ӵļ��鷽�����ʵ�鲽�������ʵ�鲽��Ϊ��ȡ������ҺA���Թ��У��μ���KSCN��Һ����Һ����ɫ���ټ���ˮ����ͨ����������Һ��ΪѪ��ɫ��֤����Һ�к����������ӣ�
�ʴ�Ϊ��ȡ������ҺA���Թ��У��μ���KSCN��Һ����Һ����ɫ���ټ���ˮ����ͨ����������Һ��ΪѪ��ɫ��
��1��ȡ��Ʒag����ȡʱʹ�õ���Ҫ��������Ϊ������ƽ���ʴ�Ϊ��������ƽ��
��2����������ϡ�����������ܽ�Ϊ�Ȼ��������Ȼ�����Һ��ͭ���ܽ⣬���˵õ�����AΪͭ����ҺA�к��е����ʳɷ�ΪFeCl2��AlCl3�������ܽ��ͨ�������ٷ������Һ�������������������ǹ��ˣ���Ҫ�õ��IJ�������Ϊ��©�������������ձ���
�ʴ�Ϊ��FeCl2��AlCl3�����ˣ�©�������������ձ���
��3����ҺA����FeCl2��AlCl3�������������������Һ�õ����ɫ����������������Һƫ��������Һ����ҺB���ɺ��ɫ����B�����ӷ���ʽΪ��Fe3++3OH-=Fe��OH��3�����ʴ�Ϊ��Fe3++3OH-=Fe��OH��3����
��4����Ʒ����Ϊag������Fe2O3�������������Ϊbg��������Ԫ���غ�������Ʒ����Ԫ����������=
| ||
| ag |
| 7b |
| 10a |
�ʴ�Ϊ��
| 7b |
| 10a |
��5����ҺA�к��е����ʳɷ�ΪFeCl2��AlCl3����֤��ҺA�к�Fe2+��������Fe3+�����������Ӻ��������ӵļ��鷽�����ʵ�鲽�������ʵ�鲽��Ϊ��ȡ������ҺA���Թ��У��μ���KSCN��Һ����Һ����ɫ���ټ���ˮ����ͨ����������Һ��ΪѪ��ɫ��֤����Һ�к����������ӣ�
�ʴ�Ϊ��ȡ������ҺA���Թ��У��μ���KSCN��Һ����Һ����ɫ���ټ���ˮ����ͨ����������Һ��ΪѪ��ɫ��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ