��Ŀ����
ij�����������ڴ���Ѳ��ֱ��ʣ�ijͬѧ�������ʵ����ȷ���䴿�ȣ�
��һ������ȡ��ƷAg��
�ڶ���������Ʒ�ܽ⣬�ò��õ���������Ҫ��______��
������������Һ�м���������������ữ��BaCl2��Һ��BaCl2��ҺҪ�������ữ��ԭ����______��
BaCl2��ҺҪ�ӹ�����ԭ����______��
���IJ������˺�ϴ�ӳ������жϳ����Ƿ�ϴ���ķ�����______
���岽����ɳ��������㣮������ɺ�ij���ΪBg�����������ƵĴ��ȼ���ʽΪ______��
��һ������ȡ��ƷAg��
�ڶ���������Ʒ�ܽ⣬�ò��õ���������Ҫ��______��
������������Һ�м���������������ữ��BaCl2��Һ��BaCl2��ҺҪ�������ữ��ԭ����______��
BaCl2��ҺҪ�ӹ�����ԭ����______��
���IJ������˺�ϴ�ӳ������жϳ����Ƿ�ϴ���ķ�����______
���岽����ɳ��������㣮������ɺ�ij���ΪBg�����������ƵĴ��ȼ���ʽΪ______��
ij�����������ڴ���Ѳ��ֱ��ʣ�������Ʒ�еijɷ�Ϊ�������ƺ������ƣ��ⶨ����������Ʒ�Ĵ��ȣ������òⶨ�������Ʊ������ɵ������ƺͱ����ӽ���������ᱵ�����������������㣻
��һ����������Ʒ������Ag����Ҫ��ƽ������
�ڶ�������Ʒ�ܽ���Ҫ���ձ��м�ˮ�ܽ⣬�ò��������Ͻ���ӿ��ܽ⣬��Ҫ�IJ�������Ϊ�ձ�����������
������������Һ�м���������������ữ��BaCl2��Һ��������Ժ�����������ӷ�Ӧ���������������ᱵ��������ʵ��ⶨ���Ȼ���������Ϊ�˸��õij�����������ӣ�
���IJ�������ϴ�ӵõ������ᱵ������ϴ�Ӹɾ��ķ��������ݳ���������ܸ����е��Ȼ��������ʵ����������Ӵ��ڻ����ӵĴ��ڣ�ʵ�����Ϊ�������ϴ�ӹ��˳�����Һ�еμ���������Һ����ϡ�������������Һ�����������ɣ�����������ϴ����
���岽�����ݳ��������������ʵ�����
�����Ƶ�����=
��142g/mol�����������Ƶ�����=Ag-
��142g/mol���õ���Ʒ�������������Ƶ���������=
��100%=��1-
����100%��
�ʴ�Ϊ���ձ�����������ֹ���������ᱵ������ʹ�����������ȫ�������ϴ�ӹ��˳�����Һ�еμ���������Һ����ϡ�������������Һ�����������ɣ�����������ϴ������1-
����100%��
��һ����������Ʒ������Ag����Ҫ��ƽ������
�ڶ�������Ʒ�ܽ���Ҫ���ձ��м�ˮ�ܽ⣬�ò��������Ͻ���ӿ��ܽ⣬��Ҫ�IJ�������Ϊ�ձ�����������
������������Һ�м���������������ữ��BaCl2��Һ��������Ժ�����������ӷ�Ӧ���������������ᱵ��������ʵ��ⶨ���Ȼ���������Ϊ�˸��õij�����������ӣ�
���IJ�������ϴ�ӵõ������ᱵ������ϴ�Ӹɾ��ķ��������ݳ���������ܸ����е��Ȼ��������ʵ����������Ӵ��ڻ����ӵĴ��ڣ�ʵ�����Ϊ�������ϴ�ӹ��˳�����Һ�еμ���������Һ����ϡ�������������Һ�����������ɣ�����������ϴ����
���岽�����ݳ��������������ʵ�����
�����Ƶ�����=
| Bg |
| 233g/mol |
| Bg |
| 233g/mol |
Ag-
| ||
| Ag |
| 142B |
| 233A |
�ʴ�Ϊ���ձ�����������ֹ���������ᱵ������ʹ�����������ȫ�������ϴ�ӹ��˳�����Һ�еμ���������Һ����ϡ�������������Һ�����������ɣ�����������ϴ������1-
| 142B |
| 233A |

��ϰ��ϵ�д�
 �����ҵ���������ϵ�д�
�����ҵ���������ϵ�д� �����̸�Ӯ����ٸ�Ч�����ܸ�ϰ���ϿƼ�������ϵ�д�
�����̸�Ӯ����ٸ�Ч�����ܸ�ϰ���ϿƼ�������ϵ�д� �����ҵ�����������ѧ���ӳ�����ϵ�д�
�����ҵ�����������ѧ���ӳ�����ϵ�д�
�����Ŀ
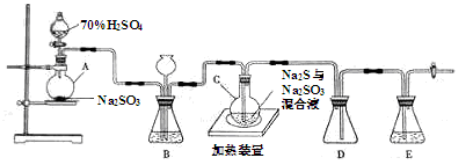
 Na2S2O3��aq�� ��III��
Na2S2O3��aq�� ��III��