��Ŀ����
8�����Ų��Ͽ�ѧ�ķ�չ�����������仯����õ���Խ��Խ�㷺��Ӧ�ã�Ϊ�������ú�������������V2O5��VOSO4�������Բ�������������Ա����������һ�������ӱ任�����շ����¹��գ������ʴﵽ91.7%�����ֺ���������ˮ�е��ܽ������±���ʾ��
| ���� | VOSO4 | V2O5 | NH4VO3 | ��VO2��2SO4 |
| �ܽ��� | ���� | ���� | ���� | ���� |
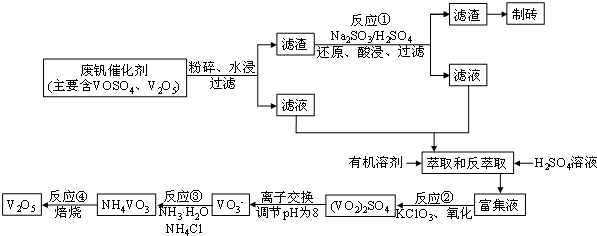
���ʴ��������⣺
��1����ҵ����V2O5ұ���������������ȼ������仯ѧ����ʽ�ɱ�ʾΪ3V2O5+10Al $\frac{\underline{\;����\;}}{\;}$6V+5Al2O3
�����ȼ��������и�������������ұ������1mol��Ӧ�Ľ����������۵�����������c������ţ�
a��Fe3O4 b��Ni2O3 c��V2O5 d��MnO2
��2����Ӧ�ٵĻ�ѧ����ʽΪV2O5+Na2SO3+2H2SO4=2VOSO4+Na2SO4+2H2O
��3��������ȡʹ��������������һ������������NH3•H2O���ѧʽ������;����ɳɱ�����
��4����Ӧ�ڵĻ�ѧ����ʽΪ��KClO3+6VOSO4+3H2O=3��VO2��2SO4+KCl+3H2SO4������Ӧ���õĻ�ԭ���뷴Ӧ���õ������������ʵ���֮��Ϊ12��7����Ϸ�������VOSO4��V2O5�����ʵ���֮��Ϊ3��2��
��5���ù��շ�Ӧ�۵ij����ʣ��ֳƳ����ʣ��ǻ��շ��Ĺؼ�֮һ��д���ò�������Ӧ�����ӷ���ʽNH4++VO3-=NH4VO3��
��6������֪Ũ�ȵ��ữ��H2C2O4��Һ�ζ���VO2��2SO4��Һ���Բⶨ��Ӧ�ں���Һ�еĺ���������֪�÷�Ӧ�Ļ�ԭ����ΪVO2+����������ΪCO2����÷�Ӧ�����ӷ���ʽΪ2VO2++H2C2O4+2H+=2 VO2++2 CO2��+2 H2O��
��7���������������һ��Ũ��������Һ�в�ͬ��̬�ķ����ӣ�V2+��V3+��VO2+��VO2+��Ϊ����������Ӧ�Ļ������ʣ��缫��Ϊ����������ܷ�ӦΪ��VO2++V3++H2O$?_{�ŵ�}^{���}$V2++VO2++2H+
�ٷŵ�ʱ��������ӦΪVO2++2H++e-�TVO2++H2O
�ڳ��ʱ��������ӦΪV3++e-�TV2+��
���� �Ϸ��������顢ˮ�������ܽ�����������ˮ��Ȼ����˵õ���������Һ�������ܽ��Ա�֪����Һ�к���VOSO4�������к���V2O5�Ȳ��������ʣ��������м����������ƺ�ϡ���ᣬ�������ƾ��л�ԭ�ԣ��ܽ�V2O5��ԭΪVOSO4��Ȼ����˵õ���������Һ������������Һ��ϲ���������أ�����ؾ��������ԣ��ܽ�VOSO4����Ϊ��VO2��2SO4��������ҺpHΪ8�Ҳ������ӽ��������õ�VO3-������Һ�м����Ȼ�泥��õ������Ե�NH4VO3������NH4VO3�õ�V2O5��
��1�����ȷ�Ӧʵ�����û���Ӧ������������������Ӧ���ɷ�������������ѧ����ʽ�����غ��������ж���������������
��2�������������ͼ��֪��Ӧ�ٵ�Ŀ�ģ���V2O5 ת��Ϊ�����Ե�VOSO4�����ڷ����ᴿ��
��3������ȡʹ�õ���������������Ҫ�ü����кͣ�
��4����ȡ��ԭ���̵IJ���֮һ��VOSO4��������Ӧ�Ļ�ѧ����ʽ���ݽ�ȡ��ԭ���̺��������̵��Ⱥ�������ѧ����ʽ�ɼ�����ô�����V2O5��VOSO4�����ʵ���֮�ȣ�
��5���ù����з�Ӧ�۵ij����ʣ��ֳƳ����ʣ��ǻ��շ��Ĺؼ�֮һ��笠����Ӻ�VO3-��Ӧ���������Ե�NH4VO3��
��6��������֪Ũ�ȵ��ữH2C2O4��Һ�ζ���VO2��2SO4��Һ����Ҫ����ΪCO2��VOSO4��������Ӧ�����ӷ��̸���������ԭ��Ӧ�Ļ��ϼ�����������۵ķ�Ԫ�ر���ԭΪ���ļ۵ķ�Ԫ�أ�H2C2O4������Ϊ������̼��д��������ƽ�ɵ����ӷ��̣�
��7��������Ӧ�ǻ�ԭ��Ӧ�����ʱ��������ӦΪ��ԭ��Ӧ����ΪV3+�õ�������V2+�ķ�Ӧ��
��� �⣺��1������������������Ӧ���ɷ�������������Ӧ����ʽΪ3V2O5+10Al $\frac{\underline{\;����\;}}{\;}$6V+5Al2O3�������ȼ��������и�������������ұ������1mol��Ӧ�Ľ��������ݵ����غ��֪��
a��3Fe3O4+8Al=9Fe+4Al2O3������1molFe������ת��$\frac{8}{9}$��3mol=$\frac{8}{3}$mol��
b��Ni2O3+2Al=2Ni+Al2O3������1molNi������ת��Ϊ2mol��
c��3V2O5+10Al=6V+5Al2O3������1molV����ת��Ϊ$\frac{10}{6}$��3mol=5mol��
d��3MnO2+4Al=3Mn+2Al2O3������1molMn����ת��Ϊ$\frac{4}{3}$��3mol=4mol��
c����ת���������۵�������࣬
�ʴ�Ϊ��3V2O5+10Al $\frac{\underline{\;����\;}}{\;}$6V+5Al2O3��c��
��2������������������ƣ�Ŀ��������������ԭ��Ӧ�����������ƻ�ԭV2O5����V2O5 ת��Ϊ�����Ե�VOSO4����Ӧ�Ļ�ѧ����ʽΪV2O5+Na2SO3+2H2SO4=2VOSO4+Na2SO4+2H2O��
�ʴ�Ϊ��V2O5+Na2SO3+2H2SO4=2VOSO4+Na2SO4+2H2O��
��3������ȡʹ�õ���������������Ҫ�ü����кͣ�������ͼ��֪��������ȡʹ��������������һ������������NH3•H2O����������ɳɱ�����
�ʴ�Ϊ��NH3•H2O��
��4���������������Լ�Na2SO3��KC1O3�����ʵ���Ϊ12mol��7mol���ֱ����������ʽ��
Na2SO3+V2O5+2H2SO4=2VOSO4+Na2SO4+2H2O KClO3+6VOSO4+3H2SO4�T3��VO��2��SO4��3+KCl+3H2O
12mol 12mol 24mol 7mol 42mol
�ô�����VOSO4�����ʵ���Ϊ42mol-24mol=18mol����˸ô�����VOSO4��V2O5�����ʵ���֮��Ϊ 18mol��12mol=3��2
�ʴ�Ϊ��3��2��
��5���ù����з�Ӧ�۵ij����ʣ��ֳƳ����ʣ��ǻ��շ��Ĺؼ�֮һ��笠����Ӻ�VO3-��Ӧ���������Ե�NH4VO3������NH4VO3������ˮ�����ø��ֽⷴӦ����VO3-�����ӷ���ʽΪ��NH4++VO3-=NH4VO3����
�ʴ�Ϊ��NH4++VO3-=NH4VO3����
��6���ⶨ��Ӧ����Һ�з��ĺ�����������֪Ũ�ȵ��ữH2C2O4��Һ�ζ���VO2��2SO4��Һ����Ҫ����ΪCO2��VOSO4�����ݻ��ϼ�������д�����ӷ���Ϊ2VO2++H2C2O4+2H+=2 VO2++2 CO2��+2 H2O��
�ʴ𰸣�2VO2++H2C2O4+2H+=2 VO2++2 CO2��+2 H2O��
��7��������Ӧ�ǻ�ԭ��Ӧ���ɵ���ܷ�Ӧ��֪�ŵ�ʱ��������ӦΪVO2++2H++e-�TVO2++H2O�����ʱ��������ӦΪ��ԭ��Ӧ����ΪV3+�õ�������V2+�ķ�Ӧ���缫��ӦΪV3++e-�TV2+��
�ʴ�Ϊ��VO2++2H++e-�TVO2++H2O��V3++e-�TV2+��
���� ����Ƚ��ۺϣ��漰������ԭ�����ӷ�Ӧ����ʽ��д����ѧ��Ӧ�ļ���ȣ�����ϰ���е���Ϣ�������Ļ�ѧ��ӦΪ���Ĺؼ���ע��ͼ�����ݵķ��������ã��ϺõĿ���ѧ���������⡢����������������Ŀ�ѶȽϴ�

 ����ȫ���ִʾ��ƪ��ϵ�д�
����ȫ���ִʾ��ƪ��ϵ�д� �����߿����ϵ�д�
�����߿����ϵ�д� �㾦�½̲�ȫ�ܽ��ϵ�д�
�㾦�½̲�ȫ�ܽ��ϵ�д�����֪Ksp[Fe��OH��3]=3.8��10-38�� Ksp[Cu��OH��2]=2��10-20��Ksp��ZnS��=1.6��10-24��
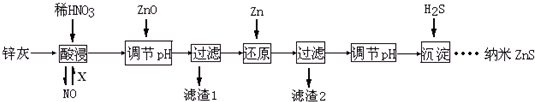
����˵������ȷ���ǣ�������
| A�� | ���ʱFeO��ϡHNO3��Ӧ�����ӷ���ʽΪ3FeO+10H++NO${\;}_{3}^{-}$�T3Fe3++NO��+5H2O | |
| B�� | �������β��ѭ�����ã������X���������O2 | |
| C�� | ����2�еijɷֺ�Zn��Fe | |
| D�� | ����Һ��Zn2+Ũ��ΪС��1.0��10-5mol•L-1ʱ�������Ϊ�������ȫ����ҪʹZn2+������ȫ����Һ��S2-Ũ��Ӧ����1.6��10-19mol•L-1 |
| A�� |  ����������ȼ�ϵ�� | B�� | 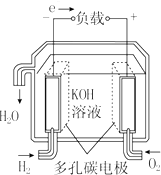 ����ȼ�ϵ�� | ||
| C�� | 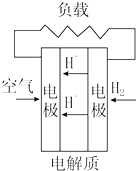 ���ӽ���Ĥȼ�ϵ�� | D�� | 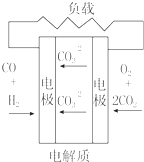 ������ȼ�ϵ�� |
| A�� | FeO | B�� | Fe3O4 | ||
| C�� | Fe2O3 | D�� | Fe3O4 ��Fe2O3����� |
| A�� | �Ȼ�ѧ����ʽδע���¶Ⱥ�ѹǿʱ����H��ʾ��״���µ����� | |
| B�� | �Ȼ�ѧ����ʽ�и�����ǰ�Ļ�ѧ����������ʾ���Ӹ�����ֻ�������ʵ��� | |
| C�� | ͬһ��ѧ��Ӧ����ѧ��������ͬ����H��ͬ����ѧ��������ͬ��״̬��ͬ����HҲ����ͬ | |
| D�� | ��ѧ��Ӧ���������ջ�ų���������μӷ�Ӧ�����ʵ����ʵ��������� |
| A�� | pH=2��pH=1��������c��H+��֮��Ϊ1��10 | |
| B�� | Na2CO3��Һ��c��Na+����c��CO32-��֮��Ϊ2��1 | |
| C�� | 0.2 mol•L-1��0.1mol/L������c ��H+��֮��Ϊ2��1 | |
| D�� | NO2����ˮʱ����������n��NO2���뱻��ԭ��n��NO2��֮��Ϊ3��1 |
 A2(g)+B2(g)�ﵽƽ��״̬�ı�־��( )
A2(g)+B2(g)�ﵽƽ��״̬�ı�־��( ) nmolA2��ͬʱ����n molAB
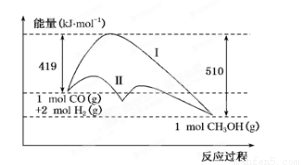
nmolA2��ͬʱ����n molAB CH3OH��g�������е������仯�������ͼ��ʾ�����ߢ�����ߢ�ֱ��ʾ��ʹ�ô�����ʹ�ô�������������������ж���ȷ����
CH3OH��g�������е������仯�������ͼ��ʾ�����ߢ�����ߢ�ֱ��ʾ��ʹ�ô�����ʹ�ô�������������������ж���ȷ����