��Ŀ����
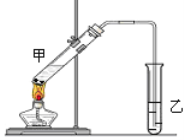
����Ŀ�������dzµ�������������Ϊ���ڴ������������������ζ��������������ʵ��������Ҳ��������ͼ��ʾ��װ����ȡ�����������ش��������⣺
��1������Ӧ���Ҵ���OΪ18O,��ͬλ��ʾ�ٷ�д����ȡ���������Ļ�ѧ��Ӧ����ʽ��
��
��2��Ũ����������ǣ��� ���� ��
��3����װ������һ�����ԵĴ����� ��
��4����Ҫ���Ƶõ������������������Ӧ���õ�ʵ������� ��
��5��ijͬѧ���ռ����������������뱥��NaHCO3��Һ�У��۲쵽���������ݲ������ɵó��Ľ����� ���ù����з�����Ӧ�����ӷ���ʽ�� ��
���𰸡���1��CH3CH218OH + CH3COOH![]() CH3CO18OC2H5 + H2O��2����
CH3CO18OC2H5 + H2O��2����
��2�� ������ ����ˮ�� ����1����
��3���������뱥��̼������Һ�� ��2���� ��4����Һ ��1����
��5����������������� ��1���� CH3COOH + HCO3�� = CH3COO�� + H2O + CO2����2����
��������
�����������1��������Ӧ����ȥ�ǻ�����ȥ��ԭ���������Ҵ�����ԭ���������������ʷ���ʽΪ��CH3CH218OH + CH3COOH![]() CH3CO18OC2H5 + H2O����2��Ũ������������Ӧ�е������������� ������ ����ˮ������3�����ܲ������뵽̼������Һ���Է�ֹ�������ʴ���Ϊ�������뱥��̼������Һ�С���4����������������ˮ����̼������Һ�ֲ㣬���Կ����÷�Һ�ķ������롣
CH3CO18OC2H5 + H2O����2��Ũ������������Ӧ�е������������� ������ ����ˮ������3�����ܲ������뵽̼������Һ���Է�ֹ�������ʴ���Ϊ�������뱥��̼������Һ�С���4����������������ˮ����̼������Һ�ֲ㣬���Կ����÷�Һ�ķ������롣
��5�����������к������ᣬ�����̼�����Ʒ�Ӧ���ɶ�����̼���塣��Ӧ�����ӷ���ʽΪ�� CH3COOH + HCO3�� = CH3COO�� + H2O + CO2����

 �߲������Ӧ��һ��ͨϵ�д�
�߲������Ӧ��һ��ͨϵ�д�����Ŀ����1��H2S��H2Se�IJ����Աȼ��ұ���
��ѧʽ | ����/nm | ���� | �е�/�� |
H2S | 1.34 | 92.3�� | -60.75 |
H2Se | 1.47 | 91.0�� | -41.50 |
��H2Se�ľ�������Ϊ__________________��
��H2S�ļ��Ǵ���H2Se��ԭ�����__________________��
���Ӿ���
����
��2����Ϊ����������A��Ԫ�أ��������ڵ�Ԫ��������33����������35������������Ԫ�صĵ縺����С�����˳��Ϊ___������Ԫ�ط��ű�ʾ��
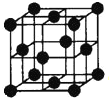
��3��һ��ͭ��Ͻ�����������������ܶѻ��ṹ���ھ����н�ԭ��λ�ڶ��㣬ͭԭ��λ�����ģ���úϽ��н�ԭ����Au����ͭԭ����Cu��������Ϊ____________1��3
�����þ���ľ�������Ϊa pm����úϽ��ܶ�
Ϊ_______________g/cm3�����г�����ʽ����Ҫ�������������٤��������ֵΪNA��
��4��Fe��CO��5��NH3��һ�������¿ɺϳ�һ�־��д��Եĵ�������
��1molFe��CO��5�����к��ЦҼ�����ĿΪ____________1��3
��
���ô��Ե����������ṹ��ͼ��ʾ���û�����Ļ�ѧʽ____________��1��3
������