��Ŀ����
��ij��Һ�н����±������е�5�����ӣ�������ˮ�ĵ��뼰���ӵ�ˮ�⣩�������ӵ����ʵ�����Ϊ1mol��
| ������ | SO42-��NO3-��Cl- |
| ������ | Fe3+��Fe2+��NH4+��Cu2+��Al3+ |
������ԭ��Һ�м���KSCN��Һ�������Ա仯��������ԭ��Һ�м�����������ᣬ���������ɣ���Һ������������䡣������ԭ��Һ�м���BaCl2��Һ���а�ɫ�������ɡ��Իش���������
��1��������ԭ��Һ�м�����������ᣬ�ټ���KSCN��Һ�������� ��
��2��ԭ��Һ�к��е��������� ��
��3����ԭ��Һ�м������������ᣬ������Ӧ�����ӷ���ʽΪ ��
��4����ԭ��Һ�м���������NaOH��Һ����ַ�Ӧ���ˡ�ϴ�ӡ����գ��������ù�����������ƽ��������Ϊ ��
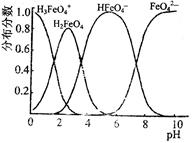
��. �����������壨FeC2O4��2H2O����̼��﮺Ͷ�������������и��·�Ӧ���Ʊ�﮵�ص��������Ϲ�������ﮣ�Li2FeSiO4��������������������������н������ط������������ͼ��ʾ��TG%��ʾ������������ռԭ��Ʒ�������İٷ�����,��ش��������⣺
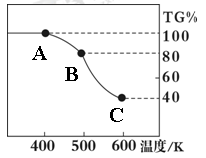
��5����������������̼Ԫ�صĻ��ϼ�Ϊ��
��6��A��B������Ӧ�Ļ�ѧ����ʽΪ ��
��7����ȷ�о�������B��Cʵ���Ƿ��������еģ�ÿһ��ֻ�ͷ�һ�����壬�ڶ����ͷŵ��������Է��������ϵ�һ���Ĵ����һ���ͷŵ����廯ѧʽΪ�� ���ͷŵڶ�������ʱ����Ӧ�Ļ�ѧ����ʽΪ ��
��16�֣�ÿ��2�֣���1����Һ�ʺ�ɫ��2�֣� ��2��Fe2+��Cu2+ ��2�֣���
��3��3Fe2++4H++NO3-��3Fe3++NO��+2H2O��2�֣� ��4��160.0g��2�֣�(д��160g������)��
��5��+3��2�֣� ��6��FeC2O4��2H2O FeC2O4 ��2H2O��2�֣�
FeC2O4 ��2H2O��2�֣�
��7��CO��2�֣� FeCO3 FeO + CO2����2�֣�
FeO + CO2����2�֣�
���������������.������Һ�м���KSCN��Һ�������Ա仯��˵��ԭ��Һ�в���Fe3+��������Һ�м�����������ᣬ���������ɣ���Һ������������䣬˵��ԭ��Һ�к���Cl-��������ֻ����NO��˵�����о��������Ե�NO3-�;��л�ԭ�Ե�Fe2+��������Һ�м���BaCl2��Һ���а�ɫ�������ɣ�˵��ԭ��Һ�к���SO42-�������ӵ����ʵ�����Ϊ1mol����˸��ݵ���غ��֪��Ӧ����Cu2+������ԭ��Һ��������������Fe2+��Cu2+����������NO3-��Cl-��SO42-��
��1������ȼ������ᣬ������������NO3-���������ԣ��ܺ;��л�ԭ�Ե�Fe2+����������ԭ��Ӧ����Ӧ�����ӷ���ʽΪ3Fe2++4H++NO3-��3Fe3++NO��+2H2O�������ٵ���KSCN��Һ����Һ�ʺ�ɫ��
��2��ԭ��Һ�к��е���������Fe2+��Cu2+��
��3���������Ϸ�����֪����ԭ��Һ�м������������ᣬ������Ӧ�����ӷ���ʽΪ3Fe2++4H++NO3-��3Fe3++NO��+2H2O��
��4��ԭ��Һ��������������Fe2+��Cu2+������ԭ��Һ�м���������NaOH��Һ����ַ�Ӧ����ˣ�ϴ�ӣ����������أ����յõ��Ĺ���Ӧ������CuO��Fe2O3��������������ӵ����ʵ�����Ϊ1mol��֪��m(CuO)��1.0mol��80g/mol��80.0g��m(Fe2O3)��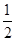 ��1.0mol��160g/mol��80.0g�����ù��������Ϊ80.0g��80.0g��160.0g��
��1.0mol��160g/mol��80.0g�����ù��������Ϊ80.0g��80.0g��160.0g��
��.��5�������������壨FeC2O4��2H2O������Ԫ�صĻ��ϼ��ǣ�2�ۣ���Ԫ���ǣ�2�ۣ�����̼Ԫ�صĻ��ϼ��ǣ�2��4��2����2����3�ۡ�
��6�����ݲ�����������Ļ�ѧʽFeC2O4��2H2O��֪�������нᾧˮ�ĺ�����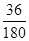 ��100%��20%������A��B������Ӧǡ����ʧȥ�ᾧˮ����˷�Ӧ�Ļ�ѧ����ʽΪFeC2O4��2H2O
��100%��20%������A��B������Ӧǡ����ʧȥ�ᾧˮ����˷�Ӧ�Ļ�ѧ����ʽΪFeC2O4��2H2O FeC2O4 ��2H2O��
FeC2O4 ��2H2O��
��7����������������FeO�ĺ�����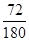 ��100%��40%������C�����ɵĹ������������������ڵڶ����ͷŵ��������Է��������ϵ�һ���Ĵ����Ե�һ���ͷŵ���CO���ڶ����ͷŵ���CO2����Ӧ�Ļ�ѧ����ʽ�ֱ���FeC2O4
��100%��40%������C�����ɵĹ������������������ڵڶ����ͷŵ��������Է��������ϵ�һ���Ĵ����Ե�һ���ͷŵ���CO���ڶ����ͷŵ���CO2����Ӧ�Ļ�ѧ����ʽ�ֱ���FeC2O4 FeCO3��CO��FeCO3
FeCO3��CO��FeCO3 FeO + CO2����
FeO + CO2����
���㣺�������ӹ���ͼ��飻����ʽ����д����ѧ���㣻���ϼ۵��ж��Լ����ʺ������йؼ�����ж�

 ���Ž�������С״Ԫϵ�д�
���Ž�������С״Ԫϵ�д�ij�����Һ�п��ܺ��е��������±���ʾ��
| ���ܴ������е������� | H+��Ag+��Mg2+��Al3+��NH ��Fe3+ ��Fe3+ |
| ���ܴ������е������� | Cl-��Br-��I-��CO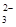 ��AlO ��AlO |
Ϊ̽����ɷ֣�����������̽��ʵ�顣
��1��̽��һ��
��ͬѧȡһ�����Ļ����Һ����������μ�������������Һ���������������ʵ���(n)��������� ������Һ�������V���Ĺ�ϵ��ͼ��ʾ��
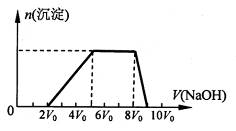
�ٸ���Һ��һ�����е���������______________�����Ӧ���ʵ���Ũ��֮��Ϊ________��һ�������ڵ���������_____________��
����д���������ٹ����з�����Ӧ�����ӷ���ʽ_____________________________��
��2��̽������
��ͬѧ������Һ�к��д�����Cl-��Br-��I-������1 L�û����Һ��ͨ��һ������Cl2����Һ��Cl-��Br-��I-�����ʵ�����ͨ��Cl2���������״�����Ĺ�ϵ���±���ʾ�� ������ش��������⣺
| Cl2���������״���� | 5.6 L | 11.2 L | 22.4 L |
| n (Cl-) | 2.5 mol | 3.0 mol | 4.0 mol |
| n (Br-) | 3.0 mol | 2.8 mol | 1.8 mol |
| n (I-) | x mol | 0 | 0 |
�ٵ�ͨ��Cl2�����Ϊ5.6 Lʱ����Һ�з�����Ӧ�����ӷ���ʽΪ_______________��
��ԭ��Һ��Cl-��Br-��I-�����ʵ���Ũ��֮��Ϊ______________________��
�������ʵ���������ͭ�Ļ�̨��24 g��600mLϡ����ǡ����ȫ��Ӧ������NO6 .72 L(��״������Ӧ�����Һ�м���l mol��L-1 NaOH��Һʹ��������ǡ�ó��������ˡ������й�˵���������
| A��������ܽ�����Һ�� c(Fe3��)�� c(Fe2+) =1��1 |
| B�������NaOH��Һ1000mL |
| C��ϡ��������ʵ���Ũ����2 mol��L-1 |
| D��������ó����ڿ����г�ּ��ȿɵù���32 g |
�ӿ���ѧ���ϲ�ã�һ����������Ȼ��������·�Ӧ��
14CuSO4��5FeS2��12H2O = 7Cu2S��5FeSO4��12H2SO4����˵����ȷ����
| A��Cu2S��������������ǻ�ԭ���� |
| B�������е�SO42����һ�������������� |
| C��5molFeS2������Ӧʱ����l0mol����ת�� |
| D��FeS2ֻ����ԭ�� |




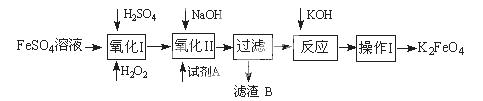
 4Fe(OH)3����8OH��+3O2��,��K2FeO4������ˮ�����е������� ��
4Fe(OH)3����8OH��+3O2��,��K2FeO4������ˮ�����е������� ��