��Ŀ����
(12��)ijУ��ѧʵ����ȤС���ڡ�̽��±�ص��ʵ������ԡ���ϵ��ʵ���з��֣���������ϡ�Ȼ�������Һ�У�����1��2����ˮ������Һ�ʻ�ɫ��
(1)������⣺Fe3����Br2��һ���������Ը�ǿ��
(2)����
�ټ�ͬѧ��Ϊ�����ԣ�Fe3��>Br2��������ʵ�������Ƿ�����ѧ��Ӧ���£�����Һ�ʻ�ɫ�Ǻ�________(�ѧʽ����ͬ)���¡�
����ͬѧ��Ϊ�����ԣ�Br2>Fe3����������ʵ�������Ƿ�����ѧ��Ӧ���£�����Һ�ʻ�ɫ�Ǻ�__________���¡�
(3)���ʵ�鲢��֤
��ͬѧΪ��֤��ͬѧ�Ĺ۵㣬ѡ������ijЩ�Լ���Ƴ����ַ�������ʵ�飬��ͨ���۲�ʵ������֤������ͬѧ�Ĺ۵�ȷʵ����ȷ�ġ���ѡ�õ��Լ���
a����̪��Һ b��CCl4 c����ˮ�ƾ� d��KSCN��Һ
���������б�����д����ͬѧѡ�õ��Լ���ʵ���й۲쵽������(�Լ������)
(4)Ӧ������չ
����������ϡ�Ȼ�������Һ�м���1��2����ˮ����Һ�ʻ�ɫ�������������ӷ�Ӧ����ʽΪ
_______________________________________________________________________��
����100 mL FeBr2��Һ��ͨ��2.24 L Cl2(��״��)����Һ����1/3��Br���������ɵ���Br2����ԭFeBr2��Һ��FeBr2�����ʵ���Ũ��Ϊ_____________________��
(1)������⣺Fe3����Br2��һ���������Ը�ǿ��
(2)����
�ټ�ͬѧ��Ϊ�����ԣ�Fe3��>Br2��������ʵ�������Ƿ�����ѧ��Ӧ���£�����Һ�ʻ�ɫ�Ǻ�________(�ѧʽ����ͬ)���¡�
����ͬѧ��Ϊ�����ԣ�Br2>Fe3����������ʵ�������Ƿ�����ѧ��Ӧ���£�����Һ�ʻ�ɫ�Ǻ�__________���¡�
(3)���ʵ�鲢��֤
��ͬѧΪ��֤��ͬѧ�Ĺ۵㣬ѡ������ijЩ�Լ���Ƴ����ַ�������ʵ�飬��ͨ���۲�ʵ������֤������ͬѧ�Ĺ۵�ȷʵ����ȷ�ġ���ѡ�õ��Լ���
a����̪��Һ b��CCl4 c����ˮ�ƾ� d��KSCN��Һ
���������б�����д����ͬѧѡ�õ��Լ���ʵ���й۲쵽������(�Լ������)
| | ѡ���Լ� | ʵ������ |
| ����1 | | |
| ����2 | | |
����������ϡ�Ȼ�������Һ�м���1��2����ˮ����Һ�ʻ�ɫ�������������ӷ�Ӧ����ʽΪ
_______________________________________________________________________��
����100 mL FeBr2��Һ��ͨ��2.24 L Cl2(��״��)����Һ����1/3��Br���������ɵ���Br2����ԭFeBr2��Һ��FeBr2�����ʵ���Ũ��Ϊ_____________________��
(2)��Br2����Fe3����(3)d����Һ�ʺ�ɫ��b��CCl4�����ɫ��
(4)��2Fe2����Br2===2Fe3����2Br������1.2 mol/L
(4)��2Fe2����Br2===2Fe3����2Br������1.2 mol/L
��

��ϰ��ϵ�д�
 ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д� ͬ����չ�Ķ�ϵ�д�
ͬ����չ�Ķ�ϵ�д�
�����Ŀ

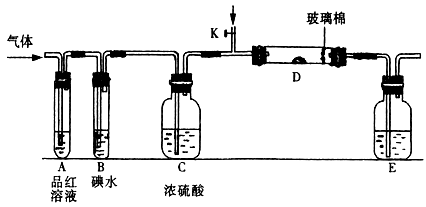
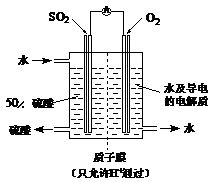
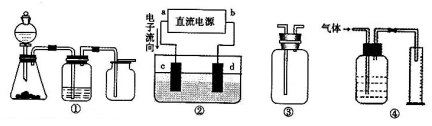
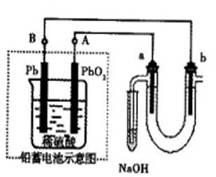


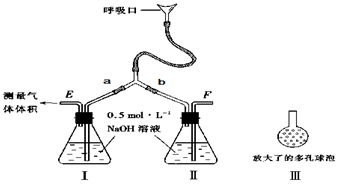
 ij�о���С�����A-D������װ�ã������ظ�ʹ�ã�����й�ʵ��
ij�о���С�����A-D������װ�ã������ظ�ʹ�ã�����й�ʵ��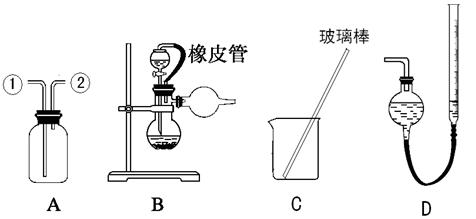

 ���������� ��
���������� ��
