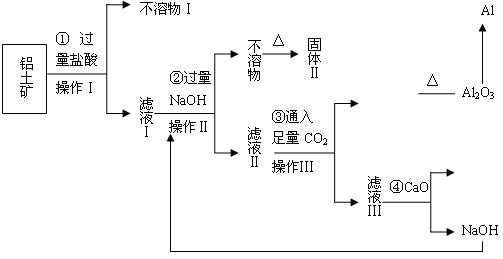
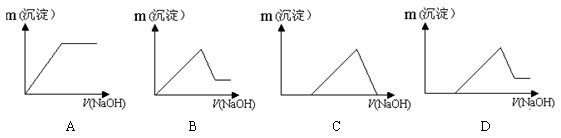
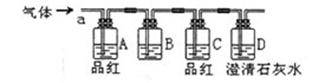
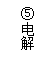��Ŀ����
��12�֣�
��֪HI��һ����ɫ���д̼�����ζ����������ˮ�����壬HI��ˮ��Һ��֮Ϊ����ᣬ��һ��ǿ�ᡣ
(1)��д��IԪ�������ڱ���λ�ã�����������������
(2)��HI����ͨ��һ������Ũ�����У������Ļ���������HI��������I2������ˮ�����⣬�������� ���塣
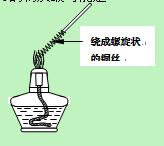
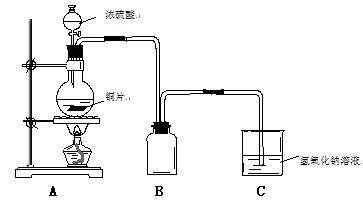
(3) С�����HIͨ��Ũ�����Ļ������ɷֽ�����֤��̽���������������ʵ��װ��ͼ

����̽����ƣ�
������ֱ�β���������װ��ҩƷ�� ��д���ƣ�
���������Ȼ�̼�����������ǣ������� ���������� ��
���������� ��
��һ��̽����
�������ϣ�������ǿ��KMnO4>HNO3>I2>SO42�����ҽ�ϡ�����������I2��
��С���������ˮ��������������ɷ�����һ��̽���������������ѡ�Լ���ѡ����ʵ��Լ���С�����ʵ�鱨�档
��ѡ���Լ���ʯ���Լ���Ʒ����Һ������KMnO4��Һ��0.1mol/L HNO3��������Һ��BaCl2��Һ
��֪HI��һ����ɫ���д̼�����ζ����������ˮ�����壬HI��ˮ��Һ��֮Ϊ����ᣬ��һ��ǿ�ᡣ
(1)��д��IԪ�������ڱ���λ�ã�����������������
(2)��HI����ͨ��һ������Ũ�����У������Ļ���������HI��������I2������ˮ�����⣬�������� ���塣
(3) С�����HIͨ��Ũ�����Ļ������ɷֽ�����֤��̽���������������ʵ��װ��ͼ

����̽����ƣ�
������ֱ�β���������װ��ҩƷ�� ��д���ƣ�
���������Ȼ�̼�����������ǣ�������
 ���������� ��
���������� ����һ��̽����
�������ϣ�������ǿ��KMnO4>HNO3>I2>SO42�����ҽ�ϡ�����������I2��
��С���������ˮ��������������ɷ�����һ��̽���������������ѡ�Լ���ѡ����ʵ��Լ���С�����ʵ�鱨�档
��ѡ���Լ���ʯ���Լ���Ʒ����Һ������KMnO4��Һ��0.1mol/L HNO3��������Һ��BaCl2��Һ
| ʵ�鷽�� | ���ܵ��������Ӧ�Ľ��� |
| ȡ�����ձ��е��ϲ���Һ��װ��A��B��֧�Թ��� | |
| | |
| | |
��12�֣�
(1) VIIA��1�֣� (2) SO2�����֣�
(2) SO2�����֣�
(3) ����ˮ����ͭ��2�֣�
�ڼ��顢���յ������������������֣�ÿ��1 �֣�
�֣�
�ۣ��𰸺�������֣�
(1) VIIA��1�֣�
 (2) SO2�����֣�
(2) SO2�����֣�(3) ����ˮ����ͭ��2�֣�
�ڼ��顢���յ������������������֣�ÿ��1
 �֣�
�֣��ۣ��𰸺�������֣�
| ʵ�鷽�� | ���ܵ��������Ӧ�Ľ��� |
| �����Թ��м�������Ʒ����Һ ��1�֣� | ��Ʒ����Һ��ɫ����ԭ�����������SO2 ����Һ����ɫ����ԭ���������û��SO2��1�֣� |
| �����Թ��еμ�����������Һ��1�֣����ٵ�������������Һ��1�֣� | ��������Һ��������ԭ�����������HI ����Һ����������ԭ���������û��HI��1�֣� |
��

��ϰ��ϵ�д�
 ������״Ԫ���Ծ�ϵ�д�
������״Ԫ���Ծ�ϵ�д�
�����Ŀ
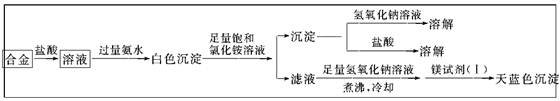

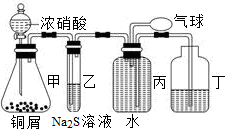
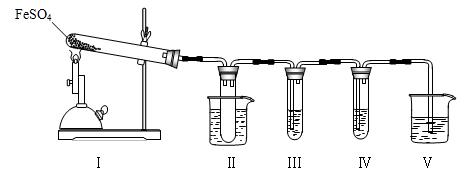
 __ ����д��ĸ��ţ��м���д��������д��д��0�֣���
__ ����д��ĸ��ţ��м���д��������д��д��0�֣��� �˶ۻ�������������________��������Ũ����δ������Ӧ��
�˶ۻ�������������________��������Ũ����δ������Ӧ��