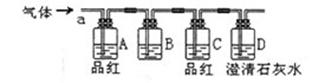
��Ŀ����
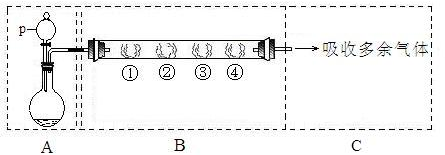
(16��) ij�о���С�����A-D������װ�ã������ظ�ʹ�ã�����й�ʵ��
ij�о���С�����A-D������װ�ã������ظ�ʹ�ã�����й�ʵ��

 ��ʵ��һ���ռ�NO���塣
��ʵ��һ���ռ�NO���塣
 ��1����װ��A�ռ�NO���壬��ȷ�IJ����� ������ţ���
��1����װ��A�ռ�NO���壬��ȷ�IJ����� ������ţ���
 a.�Ӣٿڽ���������ˮ������ b.�Ӣٿڽ�����������������
a.�Ӣٿڽ���������ˮ������ b.�Ӣٿڽ�����������������
 c.�Ӣڿڽ���������ˮ������ d..�Ӣڿڽ�����������������
c.�Ӣڿڽ���������ˮ������ d..�Ӣڿڽ�����������������
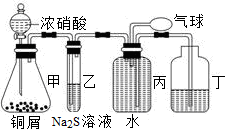
 ��ʵ�����������Ũ������KMnO4��Ӧ�õ���Cl2��
��ʵ�����������Ũ������KMnO4��Ӧ�õ���Cl2��
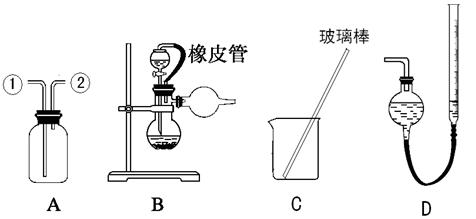
��2�������װ��A����������Cl2װ��ͼ��������ʢ��ҩƷ����ע�������������
��ʵ������Ϊ��̽����п�������ϵ�п����������w(Zn)�ͶƲ��ȣ���ѯ��֪п������ǿ�Zn��2NaOH=Na2ZnO2��H2�� �ݴˣ���ȡ���ΪS��˫���п���������������顢�Ƶ�����Ϊm1 g����NaOH��Һ���Լ����������ʵ�鷽�����������ʵ�顣
 �����ף�ͨ���������������Һ��Ӧ���ɵ����������ʵ��̽��Ŀ�ꡣ
�����ף�ͨ���������������Һ��Ӧ���ɵ����������ʵ��̽��Ŀ�ꡣ
��3�� ѡ�� �� ����������ţ�����װ�ý���ʵ�顣
ѡ�� �� ����������ţ�����װ�ý���ʵ�顣
��4�� ��ó�ַ�Ӧ���������������ΪVL����״������w(Zn)= ��
��ó�ַ�Ӧ���������������ΪVL����״������w(Zn)= ��
��5�� ����Ʋ��ȣ�����Ҫ������һ���������� ��
����Ʋ��ȣ�����Ҫ������һ���������� ��
��6�� ��װ��B�еĺ�ѹ��Һ©����Ϊ��ͨ��Һ©������������� ���ƫ����ƫС������Ӱ�족����
��װ��B�еĺ�ѹ��Һ©����Ϊ��ͨ��Һ©������������� ���ƫ����ƫС������Ӱ�족����
 �����ң�ͨ������������Ӧǰ�������ʵ��̽��Ŀ�ꡣѡ������C��ʵ�飬��������ַ�Ӧ���˳������ϴ�ӡ���ɣ��Ƶ�������Ϊm2g
�����ң�ͨ������������Ӧǰ�������ʵ��̽��Ŀ�ꡣѡ������C��ʵ�飬��������ַ�Ӧ���˳������ϴ�ӡ���ɣ��Ƶ�������Ϊm2g ��
��
��7��w(Zn)= ��
 ij�о���С�����A-D������װ�ã������ظ�ʹ�ã�����й�ʵ��
ij�о���С�����A-D������װ�ã������ظ�ʹ�ã�����й�ʵ��

 ��ʵ��һ���ռ�NO���塣
��ʵ��һ���ռ�NO���塣 ��1����װ��A�ռ�NO���壬��ȷ�IJ����� ������ţ���
��1����װ��A�ռ�NO���壬��ȷ�IJ����� ������ţ��� a.�Ӣٿڽ���������ˮ������ b.�Ӣٿڽ�����������������
a.�Ӣٿڽ���������ˮ������ b.�Ӣٿڽ����������������� c.�Ӣڿڽ���������ˮ������ d..�Ӣڿڽ�����������������
c.�Ӣڿڽ���������ˮ������ d..�Ӣڿڽ����������������� ��ʵ�����������Ũ������KMnO4��Ӧ�õ���Cl2��
��ʵ�����������Ũ������KMnO4��Ӧ�õ���Cl2����2�������װ��A����������Cl2װ��ͼ��������ʢ��ҩƷ����ע�������������
��ʵ������Ϊ��̽����п�������ϵ�п����������w(Zn)�ͶƲ��ȣ���ѯ��֪п������ǿ�Zn��2NaOH=Na2ZnO2��H2�� �ݴˣ���ȡ���ΪS��˫���п���������������顢�Ƶ�����Ϊm1 g����NaOH��Һ���Լ����������ʵ�鷽�����������ʵ�顣
 �����ף�ͨ���������������Һ��Ӧ���ɵ����������ʵ��̽��Ŀ�ꡣ
�����ף�ͨ���������������Һ��Ӧ���ɵ����������ʵ��̽��Ŀ�ꡣ��3��
 ѡ�� �� ����������ţ�����װ�ý���ʵ�顣
ѡ�� �� ����������ţ�����װ�ý���ʵ�顣��4��
 ��ó�ַ�Ӧ���������������ΪVL����״������w(Zn)= ��
��ó�ַ�Ӧ���������������ΪVL����״������w(Zn)= ����5��
 ����Ʋ��ȣ�����Ҫ������һ���������� ��
����Ʋ��ȣ�����Ҫ������һ���������� ����6��
 ��װ��B�еĺ�ѹ��Һ©����Ϊ��ͨ��Һ©������������� ���ƫ����ƫС������Ӱ�족����
��װ��B�еĺ�ѹ��Һ©����Ϊ��ͨ��Һ©������������� ���ƫ����ƫС������Ӱ�족���� �����ң�ͨ������������Ӧǰ�������ʵ��̽��Ŀ�ꡣѡ������C��ʵ�飬��������ַ�Ӧ���˳������ϴ�ӡ���ɣ��Ƶ�������Ϊm2g
�����ң�ͨ������������Ӧǰ�������ʵ��̽��Ŀ�ꡣѡ������C��ʵ�飬��������ַ�Ӧ���˳������ϴ�ӡ���ɣ��Ƶ�������Ϊm2g ��
����7��w(Zn)= ��
��1��C
��2��(4��)
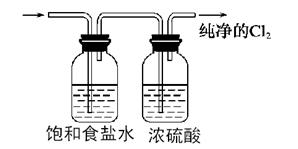
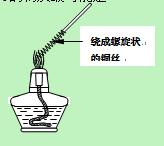
��ע������ʳ��ˮ��Ũ���ᡢ������1�֣�ͼ�еĵ��ܵij���1��
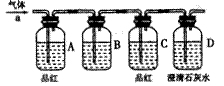
��3��B��D ����1�֣���4��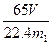 ����
����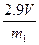 �����������𰸣���
�����������𰸣���
��5������п���ܶȣ������������𰸣���
��6��ƫ��
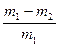 �������������𰸣���
�������������𰸣���
��2��(4��)

��ע������ʳ��ˮ��Ũ���ᡢ������1�֣�ͼ�еĵ��ܵij���1��
��3��B��D ����1�֣���4��
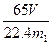 ����
����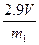 �����������𰸣���
�����������𰸣�����5������п���ܶȣ������������𰸣���
��6��ƫ��
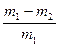 �������������𰸣���
�������������𰸣�����

��ϰ��ϵ�д�
�����Ŀ