��Ŀ����
16����1���������������У��뾶���ɴ�С������˳����S2-��S��Cl��N���ٻ�̬X��ԭ�ӽṹʾ��ͼ��

�ڻ�̬Y�ļ۵����Ų�ʽ��3s23p5
�ۻ�̬Z2-�ĵ����Ų�ͼ��

��W��̬ԭ����2���ܲ㣬����ʽ��
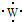
��2����֪An+��B��n+1��+��Cn-��D��n+1��-��������ͬ�ĵ��Ӳ�ṹ����A��B��C��D��ԭ�Ӱ뾶�ɴ�С��˳����A��B��D��C�����Ӱ뾶�ɴ�С��˳����D��C��A��B��ԭ�������ɴ�С��˳����B��A��C��D��
���� ��1��XΪSԭ�ӣ�YΪClԭ�ӣ�Z2-ΪS2-��WΪNԭ�ӣ����Ӳ���Խ�࣬�뾶Խ��ͬ������ԭ����������ԭ�Ӱ뾶��С��ͬ��Ԫ�ص������ӵİ뾶����ԭ�Ӱ뾶��
��2�����Ӳ�ṹ��ͬ������An+��B��n+1��+��Cn-��D��n+1��-������������������һ���ڣ�����ԭ��������С˳����B��A��C��D��ԭ�ӵ��Ӳ���Խ����ԭ�Ӱ뾶Խ��ͬһ����Ԫ�أ�ԭ�Ӱ뾶����ԭ�������������С�����Ӳ�ṹ��ͬ�����ӣ�ԭ������Խ�뾶ԽС��
��� �⣺��1���ٻ�̬X��ԭ�ӽṹʾ��ͼ�� ����XΪSԭ�ӣ�
����XΪSԭ�ӣ�
�ڻ�̬Y�ļ۵����Ų�ʽ��3s23p5����YΪClԭ�ӣ�
�ۻ�̬Z2-�ĵ����Ų�ͼ�� ��Z2-ΪS2-��
��Z2-ΪS2-��
��W��̬ԭ����2���ܲ㣬����ʽ�� ��WΪNԭ�ӣ�
��WΪNԭ�ӣ�
���Ӳ���Խ�࣬�뾶Խ����뾶��N��Cl��ͬ������ԭ����������ԭ�Ӱ뾶��С����뾶��Cl��S��ͬ��Ԫ�ص������ӵİ뾶����ԭ�Ӱ뾶����뾶��S��S2-��
���뾶��S2-��S��Cl��N��
�ʴ�Ϊ��S2-��S��Cl��N��
��2�����Ӳ�ṹ��ͬ������An+��B��n+1��+��Cn-��D��n+1��-������������������һ���ڣ�����ԭ��������С˳����B��A��C��D��ԭ�ӵ��Ӳ���Խ����ԭ�Ӱ뾶Խ��ͬһ����Ԫ�أ�ԭ�Ӱ뾶����ԭ�������������С��C��D����ͬһ���ڣ�ԭ�Ӱ뾶D��C��A��B����ͬһ���ڣ�ԭ�Ӱ뾶A��B�������⼸��ԭ�Ӱ뾶��С˳����A��B��D��C�������Ӳ�ṹ��ͬ�����ӣ�ԭ������Խ�����Ӱ뾶ԽС���������Ӱ뾶��D��C��A��B��
�ʴ�Ϊ��A��B��D��C��D��C��A��B��B��A��C��D��
���� ���⿼��ԭ�Ӱ뾶��С�Ƚϣ����ؿ��������������ȷԭ�Ӱ뾶�ȽϷ����ǽⱾ��ؼ������Ӱ뾶��С�Ƚ�Ҳ�dz�����㣬��Ŀ�Ѷ��еȣ�

 ѧ���������ν��Ͼ���ѧ������ϵ�д�
ѧ���������ν��Ͼ���ѧ������ϵ�д� Happy holiday���ּ��������ҵ�㶫���������ϵ�д�
Happy holiday���ּ��������ҵ�㶫���������ϵ�д�
| A�� | �٢� | B�� | �٢ڢ� | C�� | �٢ڢۢ� | D�� | �٢ڢۢ� |
| A�� | ������Ũ��������������棬����Ϊ���������Ũ�����жۻ� | |
| B�� | ����������Һ����������Ƥ���IJ����Լ�ƿ�� | |
| C�� | �������еμ�Ũ���ᣬ���DZ������ΪŨ���������ˮ�� | |
| D�� | Ũ���ᱣ������ɫ�����Լ�ƿ�� |
| A�� | pH=5��NaHSO3��Һ�У�c��HSO3-����c��SO32-����c��H2SO4�� | |
| B�� | ͬŨ�ȵ�������Һ�У���NH4HSO4��NH4��NH3•H2O c��NH4+����С�����˳���ǣ��ڣ��٣��� | |
| C�� | 0.1mol•L-1Na2CO3��Һ�У�c��HCO3-��=c��H2CO3��+c��H+��-C��OH-�� | |
| D�� | 0.2mol•L-1CH3COOH��Һ��0.2mol•L-1CH3COONa��Һ�������ϣ�c��CH3COO-��+c��OH-��-c��H+��=0.1mol•L-1 |
| B12�ṹ��Ԫ | SF6���� | S8���� | HCN | |
| �� �� ģ �� ʾ �� ͼ | 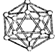 | 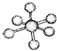 | 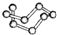 |  |
| ��ע | �۵�1873K | ������CS2 |
| A�� | ����������ԭ�Ӿ��壬�ṹ��Ԫ�к���30��B-B��������20���������� | |
| B�� | SF6���ɼ��Լ����ɵķǼ��Է��� | |
| C�� | ��̬��S8����ԭ�Ӿ��� | |
| D�� | HCN�ĽṹʽΪH-C��N |
| A�� | ����һ�����ʵ���Ũ����Һ��ʵ���У�����Ҫ��ȷ��ȡˮ������ | |
| B�� | ��Һע������ƿǰ����Ҫ�ָ������� | |
| C�� | �ý�ͷ�ιܶ��ݺ��跴����תҡ�ȣ�������Һ����ڿ̶���ʱҪС�ĵؼ�Щˮ | |
| D�� | ����ˮ����ʱ�����˿̶��ߣ��Ͽ��ý�ͷ�ι����߳����IJ��� |
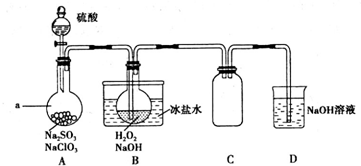
 ��ȡ����Ӧ
��ȡ����Ӧ ���Ӿ۷�Ӧ
���Ӿ۷�Ӧ