��Ŀ����
����Ŀ��ʵ������Ҫ0.5mol/L CuSO4��Һ450mL��0.5mol/L������Һ500mL��������������Һ����������ش��������⣺
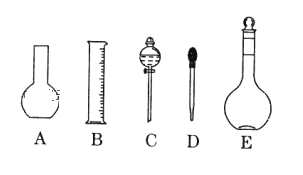
(1)��ͼ��ʾ��������������Һ�϶�����Ҫ���� _____������ţ����������� CuSO4��Һ���õ��IJ������������ձ�������������Ͳ����_______�����������ƣ���
(2)���в����У�����ƿ�����߱��Ĺ�����_________������ţ���
A.����һ�����ȷŨ�ȵı���Һ B��������Һ
C.��������ƿ������µ����������Һ�� D�����������ܽ��������
(3)����CuSO4��Һʱ�������CuSO4���壬Ӧ�ó������������Ϊ________�������CuSO4�� 5H2O�������ƣ�Ӧ�ó������������Ϊ__________������������Һʱ����Ҫȡ��������Ϊ98%���ܶ�Ϊ1.84g/cm3��Ũ��������Ϊ__________��
(4)���в�������Һ��Ũ���к�Ӱ�죿���ƫ����ƫС��������Ӱ�족��
��û�ж��ܽ����ʱ�õ����ձ�ϴ��_______________��
��ʹ��ǰ����ƿ������ˮϴ�ӣ���û�и���_______________��
�����ƵĹ�������������Һ��Ž�����_______________��
�ܶ���ʱ��С�ļ�ˮ�����������ý�ͷ�ι�����_______________��
�ݶ���ʱ���ӿ̶���_______________��
���𰸡�A��C 500mL����ƿ����ͷ�ι� B��C��D 40.0g 62.5g 13.6mL ƫС ûӰ�� ƫС ƫС ƫ��
��������
��1������������Һ��ʵ���е��������з�����
��2������m=cVM���������������
��3������ϡ���������ʵ����ʵ������������������
��4���������ƹ��������ʵı仯����Һ����ı仯����Ũ�ȵı仯��
(1)������Һʱʹ�õ�����Ϊ����ƿ���ձ�������������ͷ�ιܣ���ƽ�ȡ����Բ��õ�����ƿ�ͷ�Һ©������ѡA��C����������CuSO4��Һ���õ��IJ������������ձ�������������Ͳ����500mL����ƿ����ͷ�ιܣ�
(2)����ƿֻ����������һ�����ʵ���Ũ�ȵ���Һ������������Һ��Ҳ���ܲ�������ƿ������µ����������Һ�壬���������������ܽ�������ʣ���ѡB��C��D��
(3)����0.5mol/LCuSO4��Һ450mL�Լ�Ӧ����500mL����Ҫ������ͭ������Ϊ0.5mol/L��0.5L��160g/mol=40.0g����������ͭ���壬������Ϊ0.5mol/L ��0.5L��250g/mol=62.5g������0.5mol/L������Һ500mL��Ҫ����������Ϊ98%���ܶ�Ϊ1.84g/cm3��Ũ��������Ϊ![]() =13.6mL��
=13.6mL��
(4)��û�ж��ܽ����ʱ�õ����ձ�ϴ�ӻ�ʹ��������ʧ��Ũ��ƫС��
��ʹ��ǰ����ƿ������ˮϴ�ӣ���û�и����ʵ����Ӱ�죻
�����ƵĹ�������������Һ��Ž���������������ʧ����Ũ��ƫС��
�ܶ���ʱ��С�ļ�ˮ�����������ý�ͷ�ι���������������Һ�к������ʣ���Ũ��ƫС��
�ݶ���ʱ���ӿ̶��ߣ���Һ�������С��Ũ��ƫ��

 ���ѵ����Ԫ��ĩ���100��ϵ�д�
���ѵ����Ԫ��ĩ���100��ϵ�д� ��˼άС�ھ�100����ҵ��ϵ�д�
��˼άС�ھ�100����ҵ��ϵ�д� ��ʦָ��һ��ͨϵ�д�
��ʦָ��һ��ͨϵ�д�����Ŀ��ʵ����������480mL 0.1 mol/L��Na2CO3��Һ����ղ��ش��������⣺
(1)����480mL 0.1 mol/L��Na2CO3��Һ��
ʵ��Ӧ��Na2CO3����/g | Ӧѡ������ƿ�Ĺ��/mL |
____________ | ____________ |
(2)����ʱ������ȷ�IJ���˳����(����ĸ��ʾ��ÿ����ĸֻ����һ��)____________��
A.��30 mLˮϴ���ձ�2��3�Σ�ϴ��Һ��ע������ƿ����
B.��ȷ������Na2CO3���嵹���ձ��У��ټ�����ˮ�ܽ�
C.������ȴ����Һ�ز�����ע������ƿ��
D.������ƿ�ǽ������µߵ���ҡ��
E.���ý�ͷ�ιܼ�ˮ��ʹ��Һ����ǡ����̶�������
F.����������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶���1 cm��2 cm��
(3)�����������������������ҺŨ�Ƚ��к�Ӱ�죿(����ƫ������ƫ����������Ӱ����)
ת����Һ��δϴ���ձ��Ͳ�������ֱ�Ӷ���________��������ʱ���ӿ̶���__________��
(4)��ʵ������г������������δ�����
������ˮʱ���������˿̶�_______________
����Ŀ��ij�о�С�齫������SO2����ͨ��0.1mol��L-1��Ba��NO3��2��Һ�У��õ���BaSO4������Ϊ̽��������Һ�к�����������ͨ���SO2����С��ͻ�������¼���:
����һ����Һ�е�NO3-
���������Һ���ܽ��O2
��1����֤����һ
��С���漰ʵ����֤�˼���һ�������±��հ״���д���ʵ������
ʵ�鲽�� | ʵ������ | ���� |
ʵ��1����ʢ�в���O2��25ml0.1mol/LBaCl2��Һ���ձ��У�����ͨ�봿����SO2���� | ____ | ����һ���� |
ʵ��2����ʢ�в���O2��25ml0.1mol/LBa��NO3��2��Һ���ձ��У�����ͨ�봿����SO2���� | ____ |
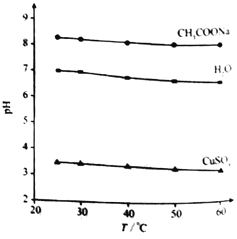
��2��Ϊ�����о��÷�Ӧ����С�黹�����������ʵ������Һ��pH��ͨ��SO2����ı仯��������ͼ
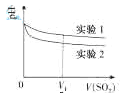
ʵ��1����ҺpH��С��ԭ����____��V1ʱ��ʵ��2����ҺpHС��ʵ��1��ԭ���ǣ������ӷ���ʽ��ʾ��_________��
��3����֤�����
�����ʵ����֤�������д��ʵ�鲽�裬Ԥ������ͽ��ۡ�
ʵ�鲽�衢Ԥ������ͽ��ۣ���Ҫ��д����������̣�____
��4�����������������Ԥ�⣺����ͬ�����£��ֱ�ͨ��������O2��KNO3��������ͬ��H2SO3��Һ����Һ����仯���Բ��ƣ�����ַ�ӳ������Һ��pHǰ��_______(����ڻ�С��)���ߣ�������________