��Ŀ����
����Ŀ��I�����ǵ����Ϻ����ḻ��һ��Ԫ�أ�������(N2H4)�����ء�ƫ������(C2H8N2)�ǵ��ij���������ڿ�ѧ����������������Ҫ��Ӧ�á�
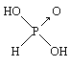
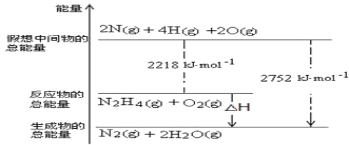
(1)��(N2H4)��һ�ָ���ȼ�ϣ�д���µĵ���ʽ________���йػ�ѧ��Ӧ�������仯����ͼ��ʾ����֪H2O(g)=H2O(l) ��H��-44kJ��mol-1��д����ȼ�յ�ȼ���ȵ��Ȼ�ѧ����ʽΪ__________��
(2)���ʹ��ƫ������(C2H8N2)��ȼ�ϣ�����������(N2O4)Ϊ��������ȼ�շ�Ӧ�ų��������ѻ������̫�գ��÷�Ӧ�Ļ�ѧ����ʽΪ____________��
II�����ĺϳ�������Ҫ�Ļ�������֮һ��
��֪��N2(g)��3H2(g)2NH3(g) ��H����92.4 kJ��mol-1
�ڼס��ҡ���������ͬ�ܱ������У�����ͬ��ʽͶ�ϣ���ʼ�¶Ⱥ��ݻ���ͬ������������±���ʾ��
���� | �� | �� | �� |
������� | ���º��� | ���Ⱥ��� | ���º�ѹ |
��Ӧ��Ͷ�� | 1molN2��3molH2 | 2molNH3 | 2molNH3 |
ƽ��ʱ������� | V�� | V�� | V�� |
��Ӧ��ƽ�ⳣ��K | K�� | K�� | K�� |
ƽ��ʱNH3��Ũ��/molL-1 | c�� | c�� | c�� |
ƽ��ʱNH3�ķ�Ӧ����/molL-1min-1 | ���� | ���� | ���� |
��ƽ��ʱ���������V��____________V����ƽ�ⳣ��K��_________K��(��>��<��=)
III������(H2NCONH2)��һ�ַdz���Ҫ�ĸߵ����ʣ��ڹ�ũҵ���������ŷdz���Ҫ�ĵ�λ��
(1)�ϳ����صĵ�һ����ӦΪ��2NH3(g)+CO2(g)H2NCOONH4(���������)(l) ��H1 ����������¡����������е�NH3��CO2�����ʵ���֮��Ϊ2��1����˵����Ӧ�ﵽ��ѧƽ��״̬����________
a�� CO2��Ũ�Ȳ��ٱ仯 b�� NH3�İٷֺ������ٱ仯 c�� ������������ܶȲ��ٱ仯 d�� ��������ƽ����Է����������ٱ仯 e�� 2��(NH3)��=�� (CO2)��
(2)����(NH2CONH2) ȼ�ϵ�ؽṹ��ͼ��ʾ���乤��ʱ�����缫��Ӧʽ�ɱ�ʾΪ��_________��

���𰸡�![]() N2H4 (g)+O2(g)=N2(g)+2H2O(l) ��H= -622kJ��mol-1 C2H8N2+2N2O4=2CO2��+3N2��+4H2O �� �� ac CO(NH2)2+H2O-6e-=CO2+N2+6H+
N2H4 (g)+O2(g)=N2(g)+2H2O(l) ��H= -622kJ��mol-1 C2H8N2+2N2O4=2CO2��+3N2��+4H2O �� �� ac CO(NH2)2+H2O-6e-=CO2+N2+6H+
��������
I����(N2H4)�ǹ��ۻ�������ݸ�˹���ɺ�ȼ���ȵĸ�����⣻ƫ������(C2H8N2)��ȼ�ϣ�����������Ϊ��������ȼ����Ҫ����CO2��N2������Ԫ���غ�֪������ˮ�����ݷ�Ӧ���������д����ʽ��
II��������Чƽ�⣬�����Ӱ��ƽ�ⳣ�������ؽ��⣻
III��(1)���ƽ��״̬����������=������0�������ʵ������ֺ㶨�����жϣ�
(2)ȼ�յ��ͨO2�ļ�Ϊ��������������������Ӧ��
I��(1)��(N2H4)�ǹ��ۻ���������ʽΪ![]() ���������仯ͼ��֪��N2H4 (g)+O2(g)=N2(g)+2H2O(g) ��H=(2218kJ��mol-1)-(2752 kJ��mol-1)=-534kJ��mol-1����֪��H2O(g)=H2O(l) ��H��-44kJ��mol-1������ݸ�˹���ɿ�֪��+����2��N2H4 (g)+O2(g)=N2(g)+2H2O(l) ��H=(-534kJ��mol-1)+(-44kJ��mol-1)��2= -622kJ��mol-1������ȼ�յ�ȼ���ȵ��Ȼ�ѧ����ʽΪN2H4 (g)+O2(g)=N2(g)+2H2O(l) ��H= -622kJ��mol-1��
���������仯ͼ��֪��N2H4 (g)+O2(g)=N2(g)+2H2O(g) ��H=(2218kJ��mol-1)-(2752 kJ��mol-1)=-534kJ��mol-1����֪��H2O(g)=H2O(l) ��H��-44kJ��mol-1������ݸ�˹���ɿ�֪��+����2��N2H4 (g)+O2(g)=N2(g)+2H2O(l) ��H=(-534kJ��mol-1)+(-44kJ��mol-1)��2= -622kJ��mol-1������ȼ�յ�ȼ���ȵ��Ȼ�ѧ����ʽΪN2H4 (g)+O2(g)=N2(g)+2H2O(l) ��H= -622kJ��mol-1��
(2) ƫ������(C2H8N2)��ȼ�ϣ�����������Ϊ��������ȼ����Ҫ����CO2��N2������Ԫ���غ�֪������ˮ����÷�Ӧ����ʽΪC2H8N2+2N2O4=2CO2��+3N2��+4H2O��
II���ס����������º��ݣ�����ȫ��Ч���ɷ�ӦN2(g)��3H2(g)2NH3(g)��֪�������������������ʵ�������Ϊ���º�ѹʱ�������������V����V��������N2(g)��3H2(g)2NH3(g) ��H����92.4 kJ��mol-1��֪���ҡ�����ȣ����ķֽ�Ϊ���ȷ�Ӧ���ұ��־��ȣ��¶Ƚ��ͣ������ֺ��£��¶ȸ����ң��¶�����ƽ�������ƶ���ƽ�ⳣ����С����K����K����
III��(1) a��CO2��Ũ�Ȳ��ٱ仯��˵����Ӧ�ﵽƽ��״̬����aѡ��
b����������麟�Һ̬����ʼ�����NH3��CO2���ʵ���֮��Ϊ2:1�����ĵ�NH3��CO2���ʵ���֮�Ⱥ�Ϊ2:1�������۷�Ӧ�Ƿ�ﵽƽ��NH3��CO2���ʵ���֮�Ⱥ�Ϊ2:1��NH3�İٷֺ���ʼ�ղ��䣬��NH3�İٷֺ������ٱ仯����˵����Ӧ�ﵽƽ��״̬����b��ѡ��
c������ƽ������������������С���ں��������£�������������ܶȼ�С��������������ܶȲ��ٱ仯��˵����Ӧ�ﵽƽ��״̬����cѡ��
d����������麟�Һ̬����ʼ�����NH3��CO2���ʵ���֮��Ϊ2:1�����ĵ�NH3��CO2���ʵ���֮�Ⱥ�Ϊ2:1�������۷�Ӧ�Ƿ�ﵽƽ��NH3��CO2���ʵ���֮�Ⱥ�Ϊ2:1����������ƽ����Է���������Ϊ17��![]() +44��
+44��![]() =26����������ƽ����Է����������ٱ仯����˵����Ӧ�ﵽƽ��״̬����d��ѡ��
=26����������ƽ����Է����������ٱ仯����˵����Ӧ�ﵽƽ��״̬����d��ѡ��
e��2��(NH3)��=�� (CO2)��ʱ�����淴Ӧ���ʲ���ȣ���Ӧû�дﵽƽ��״̬����e��ѡ��
�ʴ�Ϊac��
(2) �����ڸ�������ʧ���ӵ�������Ӧ���ɶ�����̼�͵����������������õ����ӷ�����ԭ��Ӧ����ˮ�������缫��ӦΪO2+4H++4e-=2H2O�������缫��ӦΪ��CO(NH2)2+H2O-6e-=CO2+N2+6H+��

����Ŀ����������(ClNO)���л���ϳ��е���Ҫ�Լ�������NO��Cl2�ڳ��³�ѹ�·�Ӧ�õ���ClNO �����������£���ɫ���壬�۵㣺-59.6�棬�е㣺-6.4�棬��ˮ��ˮ�⡣ij�о���ѧϰС�������������(ClNO)��������ͨ�������ȡ�������ȣ����������ʵ��װ�á���
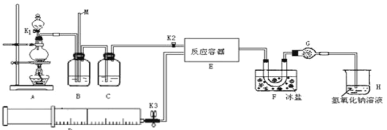
�Իش��������⣺
(1)д��ʵ��������װ��A�Ʊ�Cl2�����ӷ�Ӧ����ʽ__________��
(2)װ��B�������� ��__________����____________��
(3)����Aװ����ȡNO���壬B��Ӧ��ʢ��_________��
(4)ΪʹNO��Cl2ǡ����ȫ��Ӧ����ClNO����������E��ͨ��NO��Cl2������������ٱ�Ϊ___��
(5) �������A��B��C����ɵ�ʵ��װ�������ԵIJ���_________��
(6)װ��H����β������ͬѧ��Ϊβ���е�ij�����岻����ȫ�����ա�Ϊ�˳������β�����ɽ�β����________________ͬʱͨ��NaOH��Һ�С�
(7)��֪��ClNO��H2O��Ӧ����HNO2��HCl��
�����ʵ��֤�� HNO2�����____________��(���ṩ���Լ���1 molL-1HCl�� 1 molL-1HNO2��Һ�� NaNO2��Һ����ɫʯ����ֽ����ɫʯ����ֽ)��
��ͨ������ʵ��ⶨClNO��Ʒ�Ĵ��ȡ�ȡF������Һ��3.0 g ����ˮ�����Ƴ�250 mL ��Һ��ȡ��25.00 mL��Ʒ������ƿ�У���0.20 molL-1 AgNO3����Һ�ζ����յ㣬���ݱ���������Ϣ��Ӧѡ��____________��ָʾ�����ζ��յ��������______��
���� | Ag2CrO4 | AgCl | AgI | Ag2S |
��ɫ | ש��ɫ | ��ɫ | ��ɫ | ��ɫ |
Ksp | 1��10-12 | 1.56��10-10 | 8.3��10-17 | 6.3 ��10-50 |
���ı�AgNO3��Һ�����Ϊ20.00ml����������(ClNO)����������Ϊ________��
����Ŀ������ʵ�������ͼ����Ϣ���ܳ��˵����Ӧ�Ļ�ѧ��Ӧ�Ƿ��ȷ�Ӧ����
A | B | C | D | |
ͼʾ |
|
|
|
|
��� ��Ϣ | �¶ȼƵ�ˮ������������ | ��Ӧ������������������������ | ��Ӧ��ʼ�״�Һ������Ҵ�Һ�� | ��Ӧ��ʼ����Ͳ���������ƶ� |
A.AB.BC.CD.D