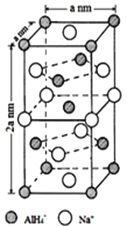
��Ŀ����
����Ŀ����ͼ��Ԫ�����ڱ���һ���֣�����������ĸ�ֱ����һ��Ԫ�أ�

�Իش��������⣺
��1������Ԫ���У�����d��Ԫ�ص���________����Ԫ�ط��ţ���h�����ڱ���λ����_________��
��2����e��f��ԭ�Ӹ���1��1�γɵľ����У���ѧ������Ϊ______________��
��3��Ԫ��k�Ļ�̬ԭ�ӵ����Ų�ʽΪ__________������c�ĺ�������Ų�ͼ��_____________��
��4��Ԫ��c�ĵ縺��______Ԫ��g�ĵ縺�ԣ����������=��������������Ԫ��b��c��e�ĵ�һ�������ɴ�С��˳����___________________����Ԫ�ط��ű�ʾ����
��5����ҵ��ұ��gԪ�صĵ��ʵĻ�ѧ����ʽ_______________________________��f�� h����Ԫ������������Ӧ��ˮ���ﷴӦ�Ļ�ѧ����ʽ��___________________��
���𰸡�Ti��Fe�������ڵ�V����A�����Ӽ��ͣ��Ǽ��ԣ����ۼ�1s22s22p63s23p63d64s2![]() ��N>O>C2C+SiO2
��N>O>C2C+SiO2![]() 2CO��+SiNaOH+HClO4=NaClO4+H2O
2CO��+SiNaOH+HClO4=NaClO4+H2O
��������
��Ԫ�������ڱ���λ�ã���֪aΪH��bΪC��cΪN��eΪO��fΪNa��gΪSi��hΪCl��jΪTi��kΪFe��
(1)d��Ԫ�ذ�����B�填��B�塢������Ԫ�أ���3��10��Ԫ��(��ϵԪ�ء��ϵԪ�س���)������Ԫ����Ti��Fe����d��Ԫ�أ�����h��λ�ã���֪�䴦�ڵ������ڵ���A�壻�ʴ�Ϊ��Ti��Fe���������ڵ���A�壻
(2)��e��f��ԭ�Ӹ���1��1�γɵľ���Ϊ�������ƣ����к������Ӽ��ͷǼ��Թ��ۼ����ʴ�Ϊ�����Ӽ���(�Ǽ���)���ۼ���
(3)FeԪ�ػ�̬ԭ�ӵ����Ų�ʽΪ��1s22s22p63s23p63d64s2��Nԭ�ӵĺ�������Ų�ʽΪ1s22s22p3����������Ų�ͼ��![]() ���ʴ�Ϊ��1s22s22p63s23p63d64s2��
���ʴ�Ϊ��1s22s22p63s23p63d64s2�� ![]() ��
��
(4)ͬ�������϶��µ縺�Լ�С���ʵ縺��C��Si��ͬ����������ҵ�һ�����ܳ��������ƣ�NԪ��ԭ��2p�������3�����ӣ�Ϊ�����ȶ�״̬�������ϵͣ���һ�����ܸ���ͬ��������Ԫ�أ��ʵ�һ������N��O��C���ʴ�Ϊ������N��O��C��
(5)��ҵ��ͨ��̼��ԭ��������ұ���裬��Ӧ�Ļ�ѧ����ʽΪ2C+SiO2![]() 2CO��+Si��f�� h����Ԫ������������Ӧ��ˮ����ֱ�Ϊ�������ƺ����ᣬ��Ӧ�Ļ�ѧ����ʽΪNaOH+HClO4=NaClO4+H2O���ʴ�Ϊ��2C+SiO2
2CO��+Si��f�� h����Ԫ������������Ӧ��ˮ����ֱ�Ϊ�������ƺ����ᣬ��Ӧ�Ļ�ѧ����ʽΪNaOH+HClO4=NaClO4+H2O���ʴ�Ϊ��2C+SiO2![]() 2CO��+Si��NaOH+HClO4=NaClO4+H2O��
2CO��+Si��NaOH+HClO4=NaClO4+H2O��

����Ŀ�����ǵ�IIIA��Ԫ�أ��������ڼ���������������ַǽ�����Ӧ��ijͬѧ��������������������Ӧ�Ʊ����Ȼ�����֪BC13�ķе�Ϊ12.5 �� ���۵�Ϊ-107.3 �棬��ˮ���ҷ�Ӧ��������������ᡣ��ͬѧѡ����ͼ��ʾ�IJ���װ�ã������ظ�ѡ�ã�����ʵ�飬��ش��������⣺

��1��A�з�Ӧ�����ӷ���ʽΪ__________________��
��2��ͼ��g�ܵ�������______________________________________��
��3��װ�õ�����˳������ΪA�� �� �� ��E��D��F��____________��E��Fװ�ü�����Dװ�õ�������____________________________________________________________��
��4��ֹͣʵ��ʱ����ȷ��ʵ�������______________________________________________________________________________________________________________��
��5��������(H3BO3)ΪһԪ���ᣬ��������NaH2BO3Ϊ_____������Ρ�����ʽ�Ρ���ʽ�Ρ�����
��6��ʵ����ɺ�ijͬѧ��F�У���Һ����0.05mol/LNaC1O�� 0.05mol/LNaCl��0.1mol/LNa0H���μ�Ʒ����Һ��������Һ��ɫ�������ʵ��̽����Һ��ɫ��ԭ�����ڱ��пո��������ݣ����ʵ�鷽����
ʵ����� | 0.1mol/LNaClO��Һ/mL | 0.1mol/LNaCl��Һ/mL | 0.2mol/LNaOH��Һ/mL | H2O /mL | Ʒ�� ��Һ | ���� |
�� | 5.0 | 0 | 0 | x | 4�� | �Ͽ���ɫ |
�� | 0 | 5.0 | 5.0 | 0 | 4�� | ����ɫ |
�� | 5.0 | 0 | 5.0 | 0 | 4�� | ������ɫ |
��x=_______�����ۣ�________________________________________________��