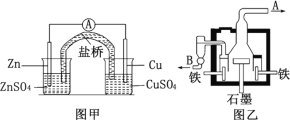
��Ŀ����
����Ŀ�������£�H2C2O4�ĵ���ƽ�ⳣ��Ka1= 5.9 ��10 -2��K a2= 6.4 ��10 -5����0.100 0 molL-1 NaOH��Һ�ζ�20.00 mL 0.100 0 molL-1H2C2O 4��Һ��������ͼ��ʾ������仯���Բ���)������˵����ȷ����
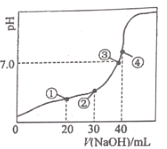
A.�ζ������У��� pH=4 ʱ�����ڣ�c(H+)+c(Na+ )=c(OH- )+c(![]() )+c(
)+c(![]() )
)
B.��١��ۡ�����ʾ��Һ�У������ʾ��Һˮ�ĵ���̶����
C.�����ʾ��Һ��3c(![]() )+2c(
)+2c(![]() )+c(H2C2O4)=0.l molL-1
)+c(H2C2O4)=0.l molL-1
D.�ζ������п��ܳ��֣�c(Na+)��c(![]() )= c(
)= c(![]() )��c(OH-)��c(H+)
)��c(OH-)��c(H+)
���𰸡�C
��������
A���ζ������У��� pH=4ʱ��Ϊ������Һ�����ڵ���غ㣺c(H+)+c(Na+ )=c(OH- )+2c(![]() )+c(
)+c(![]() )����A����
)����A����
B�������Һ�����ԣ�����ˮ�ĵ��룬��۶�Ӧ����Һ�����ԣ������ʾ��Һ��������������40mL������ǡ����ȫ��Ӧ����Һ�е�����Ϊ�����ƣ����������ˮ��ʹ��Һ�Լ��ԣ��ٽ�ˮ�ĵ��룬������ʾ��Һˮ�ĵ���̶����B����
C��ԭ������Һ�д��������غ㣺c(![]() )+c(
)+c(![]() )+c(H2C2O4)=0.l molL-1����۶�Ӧ����Һ�����ԣ�c(H+)=c(OH-)�����ݵ���غ㣺c(H+)+c(Na+)=c(OH-)+2c(
)+c(H2C2O4)=0.l molL-1����۶�Ӧ����Һ�����ԣ�c(H+)=c(OH-)�����ݵ���غ㣺c(H+)+c(Na+)=c(OH-)+2c(![]() )+c(
)+c(![]() )���ɵ�c(Na+)=2c(
)���ɵ�c(Na+)=2c(![]() )+c(
)+c(![]() )������ʱ���������������Һ�����ΪV��3c(
)������ʱ���������������Һ�����ΪV��3c(![]() )+2c(
)+2c(![]() )+c(H2C2O4)=2c(
)+c(H2C2O4)=2c(![]() )+c(
)+c(![]() )+c(
)+c(![]() )+c(
)+c(![]() )+c(H2C2O4)= c(Na+)+ c(
)+c(H2C2O4)= c(Na+)+ c(![]() )+c(
)+c(![]() )+c(H2C2O4)=
)+c(H2C2O4)=![]() =
=![]() =0.l molL-1����C��ȷ��
=0.l molL-1����C��ȷ��
D����c(OH-)��c(H+)����Һ�Լ��ԣ������֮�����������������Һ�ɷ��ϣ�c(Na+)��c(![]() )����
)����![]() ������
������![]() ��ˮ�⣬�ҼӼ�����
��ˮ�⣬�ҼӼ�����![]() ��ˮ�⣬����Һ��ʼ��c(
��ˮ�⣬����Һ��ʼ��c(![]() )��c(
)��c(![]() )����������ȣ���D����
)����������ȣ���D����
��ѡC��

 Сѧ��10���ӿ������100��ϵ�д�
Сѧ��10���ӿ������100��ϵ�д�����Ŀ�����÷Ͼ�п��Ƥ�Ʊ�����Fe3O4�������Ӽ�������ZnO���Ʊ�����ͼ���£�

��֪��Zn���������������Al����������������ơ���ش��������⣺
��1����NaOH��Һ�����Ͼ�п��Ƥ��������_________________________________________��
A��ȥ������ | B���ܽ��п�� | C��ȥ������ | D���ۻ� |
��2��������ҺA��pH�ɲ���Zn(OH)2������Ϊ�Ƶ�ZnO����������������______________��
��3������ҺB�Ƶ�Fe3O4�������ӵĹ����У������ͨ��N2��ԭ����___________________��
��4��Fe3O4���������ܷ��ü�ѹ���˷�ʵ�ֹ�Һ���룿____________������������������������������_________________________________________________��
��5�����ظ���ط���һ��������ԭ�ζ������ɲⶨ����Fe3O4�еĶ�������������������Ũ��Ϊ0.01000 mol��L��1��K2Cr2O7����Һ250 mL��Ӧȷ��ȡ_______g K2Cr2O7(����4λ��Ч���֣���֪M(K2Cr2O7)��294.0 g��mol��1)��
���Ƹñ���Һʱ�����������в���Ҫ�õ�����_____________�����ñ�ű�ʾ����
�ٵ�����ƽ ���ձ� ����Ͳ �ܲ����� ������ƿ ��ͷ�ι� ����Һ��
��6���ζ������У�����ζ�ǰװ��K2Cr2O7����Һ�ĵζ��ܼ��첿�������ݣ����ζ�������������ʧ����ⶨ�����__________������ƫ��������ƫС����������������
����Ŀ��I.�������Ƶ�������Ӧ:2Na2SO3 (aq) +O2(aq)=2Na2SO4(aq) H=x kJ/mol���䷴Ӧ�������ܽ���Ũ��Ӱ��,��Ϊ��������ƶ���������Ρ�
��1����֪O2(g) ![]() O2(aq) H=y kJ/mol��Na2SO3 ��Һ��O2(g)��Ӧ���Ȼ�ѧ����ʽΪ___________________��
O2(aq) H=y kJ/mol��Na2SO3 ��Һ��O2(g)��Ӧ���Ȼ�ѧ����ʽΪ___________________��
��2��291.5 Kʱ,1.0 L��Һ��Na2SO3��ʼ���ֱ�Ϊ4��6��8��12 mmol,�ܽ���Ũ�ȳ�ʼֵΪ9.60 mg/L,ÿ5 s��¼�ܽ���Ũ��,ʵ������ͼ��ʾ����Na2SO3��ʼ��Ϊ12 mmol,����20 s�ܽ���Ũ�Ƚ�Ϊ6.40 mg/L,��0��20s��Na2SO3��ƽ����Ӧ����Ϊ_______mol/(L��s)��
��3��Ϊȷ��ƶ�������ʷ���v=k��ca(SO32-)��cb(O2)�е�a��b��ֵ(ȡ����),����ʵ�����ݡ�
c(Na2SO3)��103 | 3.65 | 5.65 | 7.65 | 11.65 |
v��106 | 10.2 | 24.4 | 44.7 | 103.6 |
�ٵ��ܽ���Ũ��Ϊ4.0 mg/Lʱ,c(SO32-)��������ֵ��ϵ���(��)��ʾ����a=____��
�ڵ��ܽ���Ũ��С��4.0mg/Lʱ,ͼ�����߽�Ϊֱ��,Na2SO3�����������ܽ���Ũ����,��b=_______��
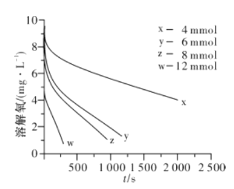
��4�������β�ͬ�¶ȵ����ʳ���֮�����(��)��ʾ����֪![]() ,RΪ������Ea(������)_____ (���������)Ea(ƶ����)��
,RΪ������Ea(������)_____ (���������)Ea(ƶ����)��
��Ӧ�� | ���ʷ��� |
|
������ | v=k��c (SO32-)��c (O2) | 1.47 |
ƶ���� | v=k��ca (SO32-)��cb(O2) | 2.59 |
II. ��5�����ݻ��̶����ܱ�������,��ʼ����0.2 mol SO2��0.1 mol O2,��Ӧ��ϵ��ʼ��ѹǿ0.1MPa����Ӧ��һ���¶��´ﵽƽ��ʱSO2��ת����Ϊ90%���÷�Ӧ��ѹǿƽ�ⳣ��Kp=________ ( ��ѹ=��ѹ�����ʵ�������)(д��λ)��
��6������ԭ���ԭ��,Ҳ����SO2��O2���Ʊ�����,�õ���ö�ײ������缫����д���õ�ظ�����Ӧʽ_________________________��