��Ŀ����
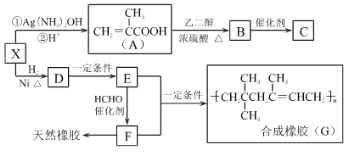
����Ŀ������ҵ�ǹ��õ���Ҫ������ҵ֮һ�����ִ����������¹�ҵ��ҽ����ҵ���й㷺Ӧ�á���ͼ�������ϳ���G��ҽ�ø߷��Ӳ���C��·��ͼ����֪B�ķ���ʽΪC6H10O3����ش��������⣺
(1)X�еĺ���������������___��X�ĺ˴Ź���������___��塣
(2)A��B�ķ�Ӧ������___��
(3)C�Ľṹ��ʽ��___��
(4)X����������Ӧ�Ļ�ѧ����ʽΪ___��
(5)д��E��F�Ļ�ѧ��Ӧ����ʽ___��
(6)A�ж���ͬ���칹�壬�������������Һ���̼̼˫���Ĺ���___��(���������칹)��
(7)��֪����![]() ��
��![]() +SOCl2��
+SOCl2��![]() +SO2+HCl���뽫������
+SO2+HCl���뽫������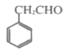 Ϊԭ���Ʊ�
Ϊԭ���Ʊ� �ĺϳ�·������ͼ��������___(���Լ�����)��
�ĺϳ�·������ͼ��������___(���Լ�����)��
![]()
���𰸡�ȩ�� 3 ������Ӧ 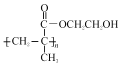 CH2=C(CH3)CHO+2Ag(NH3)2OH
CH2=C(CH3)CHO+2Ag(NH3)2OH![]() CH2=C(CH3)COONH4+2Ag��+3NH3+H2O
CH2=C(CH3)COONH4+2Ag��+3NH3+H2O ![]() +HCHO
+HCHO![]()
![]() +H2O 5
+H2O 5 

��������
����X��A�ķ�Ӧ������֪���ù���ΪX��ȩ�����������Ȼ��Ĺ��̣�����X�Ľṹ��ʽΪ![]() ��X�������ӳ�����D��̼̼˫����ȩ���е�̼��˫�����ɼӳɣ���DΪ
��X�������ӳ�����D��̼̼˫����ȩ���е�̼��˫�����ɼӳɣ���DΪ![]() ��F���Ժϳ���Ȼ������FΪ
��F���Ժϳ���Ȼ������FΪ![]() �����F��G�Ľṹ��ʽ��֪EΪ
�����F��G�Ľṹ��ʽ��֪EΪ![]() ������D��EΪ�ǻ�����ȥ��Ӧ��A���Ҷ�������������Ӧ����B������BΪ
������D��EΪ�ǻ�����ȥ��Ӧ��A���Ҷ�������������Ӧ����B������BΪ![]() ��B�к���̼̼˫���������Ӿ۷�Ӧ����CΪ
��B�к���̼̼˫���������Ӿ۷�Ӧ����CΪ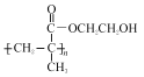 ��
��
(1)XΪ![]() ���京��������Ϊȩ��������3�ֻ�������ԭ�ӣ����Ժ˴Ź���������3��壻
���京��������Ϊȩ��������3�ֻ�������ԭ�ӣ����Ժ˴Ź���������3��壻
(2)A��BΪ�Ȼ����ǻ���������Ӧ(ȡ����Ӧ)��
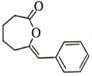
(3)���ݷ�����֪C�Ľṹ��ʽΪ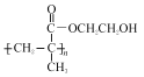 ��
��
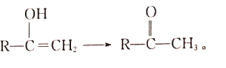
(4)X����������Ӧ�ķ���ʽΪCH2=C(CH3)CHO+2Ag(NH3)2OH![]() CH2=C(CH3)COONH4+2Ag��+3NH3+H2O��
CH2=C(CH3)COONH4+2Ag��+3NH3+H2O��
(5)EΪ![]() ��FΪ
��FΪ![]() �����Է�Ӧ����ʽΪ
�����Է�Ӧ����ʽΪ![]() +HCHO
+HCHO![]()
![]() +H2O��
+H2O��
(6)A��ͬ���칹�������������Һ���̼̼˫�����У�CH2=CHCOOCH3��CH2=CHCH2OOCH��CH=C(CH3)OOCH��CH3CH=CHOOCH��CH2=CHOOCCH3����5�֣�
(7)�Ա� ��
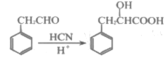
��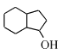 �Ľṹ��֪����Ҫ�������ӳɣ������γ�һ������������Ŀ������Ϣ��֪
�Ľṹ��֪����Ҫ�������ӳɣ������γ�һ������������Ŀ������Ϣ��֪![]() ���Ժ�SOCl2�γ�
���Ժ�SOCl2�γ�![]() �����ýṹ����ȡ�������ϵ���ԭ�ӣ��ݴ˿����γ���һ����״�ṹ���ٽ���ǻ����Է�����ȥ��Ӧ����˫�����ʻ����Ժ������ӳ������ǻ�����֪�ϳ�·��ӦΪ
�����ýṹ����ȡ�������ϵ���ԭ�ӣ��ݴ˿����γ���һ����״�ṹ���ٽ���ǻ����Է�����ȥ��Ӧ����˫�����ʻ����Ժ������ӳ������ǻ�����֪�ϳ�·��ӦΪ 
 ��
��

 �Ƹ�С״Ԫ�������������ϵ�д�
�Ƹ�С״Ԫ�������������ϵ�д� ����һ������ܼƻ�ϵ�д�
����һ������ܼƻ�ϵ�д�