��Ŀ����
����Ŀ���Ҵ�����Ҫ�Ļ���ԭ�Ϻ�Һ��ȼ�ϣ������������з�Ӧ��ȡ�Ҵ���2CO2(g) + 6H2(g) ![]() CH3CH2OH(g) + 3H2O(g)
CH3CH2OH(g) + 3H2O(g)
��1��д���÷�Ӧ��ƽ�ⳣ������ʽ��K��_________________________��
��2����˵����CO2Ϊԭ�Ϻϳ��Ҵ����ŵ���_____________________��ֻҪ��д��һ������
��3����һ��ѹǿ�£���ø÷�Ӧ��ʵ���������±���
| 500 | 600 | 700 | 800 |
1.5 | 45 | 33 | 20 | 12 |
2.0 | 60 | 43 | 28 | 15 |
3.0 | 83 | 62 | 37 | 22 |
�� �÷�Ӧ��___________��Ӧ������ȡ����ȡ�����
�� һ�����������������̼��[n(H2)/n(CO2)]����CO2��ת����______________�����������С�������䡱��
��4��һ���Ҵ�ȼ�ϵ���з����Ļ�ѧ��ӦΪ����������Һ���Ҵ�������������ˮ�Ͷ���
��̼���õ�صĸ�����ӦʽΪ��_______________________________________��
��5��25����101 kPa�£�H2(g)��C2H4(g)��C2H5OH(l)��ȼ���ȷֱ���285.8 kJ �� mol��1��1411.0 kJ �� mol��1��1366.8 kJ �� mol��1����д����C2H4(g)��H2O(l)��Ӧ����C2H5OH(l)���Ȼ�ѧ����ʽ____________________________________________________��
���𰸡� c(CH3CH2OH)c3(H2O)/c2(CO2)c6(H2) �����������ã������ڻ��� ���� ���� C2H5OH��12e-+3H2O=2CO2��+12H+ C2H4(g)+H2O(l)=C2H5OH(l) ��H=��44.2kJ/mol
��������(1)2CO2(g)+6H2(g)=CH3CH2OH(g)+3H2O(g)�����ݷ�Ӧ��ѧ����ʽ����ϻ�ѧƽ�ⳣ���ĸ�����ʽд��ƽ�ⳣ������ʽΪ�� 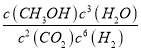 ���ʴ�Ϊ��
���ʴ�Ϊ�� 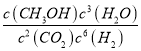 ��
��
(2)��CO2Ϊԭ�Ϻϳ��Ҵ������ٶ�����̼��ɵ�����ЧӦ�����������ã������ڻ������ʴ�Ϊ�����������ã������ڻ�����
(3)����ͼ�������жϣ����¶����ߣ�������̼ת���ʼ�С��˵��ƽ��������У����������ȷ�Ӧ���������Ƿ��ȷ�Ӧ���ʴ�Ϊ�����ȣ�
��һ�������£��������̼��[n(H2)/n(CO2)]��ͼ�����ݷ�����ͬ�¶��£�������̼ת�������ʴ�Ϊ������
(4)���������£��Ҵ��ڸ�������������Ӧ���ɶ�����̼������ӦΪC2H5OH-12e-+3H2O=2CO2+12H+���ʴ�Ϊ��C2H5OH+3H2O-12e-�T2CO2+12H+��
(5)��֪H2(g)��C2H4(g)��C2H5OH(l)��ȼ���ȷֱ���-285.8kJ/mol��-1411.0kJ/mol��-1366.8kJ/mol�����У���H2(g)+ ![]() O2(g)=H2O(l)��H=-285.8kJ/mol����C2H4(g)+2O2(g)=2H2O(l)+2CO2(g)��H=-1411.0kJ/mol����C2H5OH(l)+2O2(g)=3H2O(l)+2CO2 (g)��H=-1366.8kJ/mol�����ݸ�˹���� ������-�ۿɵã�C2H4(g)+H2O(l)=C2H5OH(l) ��H=-44.2kJ/mol���ʴ�Ϊ��C2H4(g)+H2O(l)�TC2H5OH(l)����H=-44.2 kJ/mol-1��
O2(g)=H2O(l)��H=-285.8kJ/mol����C2H4(g)+2O2(g)=2H2O(l)+2CO2(g)��H=-1411.0kJ/mol����C2H5OH(l)+2O2(g)=3H2O(l)+2CO2 (g)��H=-1366.8kJ/mol�����ݸ�˹���� ������-�ۿɵã�C2H4(g)+H2O(l)=C2H5OH(l) ��H=-44.2kJ/mol���ʴ�Ϊ��C2H4(g)+H2O(l)�TC2H5OH(l)����H=-44.2 kJ/mol-1��

 ���Ӣ��������ϵ�д�
���Ӣ��������ϵ�д�


