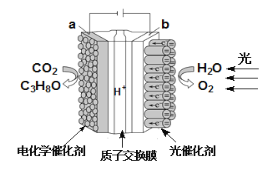
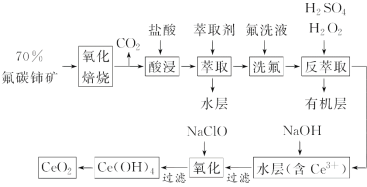
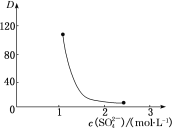
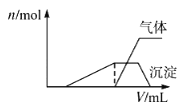
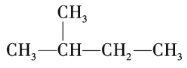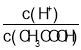��Ŀ����
����Ŀ�������£������й���Һ��˵����ȷ���ǣ� ��
A.pH��ȵĢ�NH4Cl��NH4Al(SO4)2��NH4HSO4��Һ��NH4��Ũ�ȵĴ�С˳��Ϊ��>��>��
B.�����£�pHΪ5���Ȼ����Һ��pHΪ5�Ĵ�����Һ��ˮ�ĵ���̶���ͬ
C.HA�ĵ��볣��Ka=4.93��10-10�����Ũ�ȵ�NaA��HA�����Һ�У�c(Na��)>c(HA)>c(A��)
D.��֪����ͬ����������HF>CH3COOH�������ʵ���Ũ����ȵ�NaF��CH3COOK��Һ�У�c(Na��)��c(F-)>c(K��)��c(CH3COO-)
���𰸡�A
��������
A������������笠�����ˮ�⣬NH4HSO4��Һ��ǿ���ԣ�NH4Al(SO4)2������������ˮ�������ԣ�Ҫʹ��������Һ��pH��ȣ���NH4Cl��NH4Al(SO4)2��Һ�������ӵ�ˮ��̶���ȣ��������Ũ����С������NH4��Ũ�ȵĴ�С˳��Ϊ��>��>�ۣ���A��ȷ��
B���Ȼ����Һ��笠�ˮ��ٽ�ˮ�ĵ��룬������Һ�д����������ˮ�ĵ��룬��pH��ͬʱ����Һ��ˮ�ĵ���̶Ȳ�ͬ����B����
C��Kh��![]() ��Ka����֪�����Һ���ε�ˮ���������ĵ��룬���Ũ�ȵ�NaA��HA�����Һ�У� c(HA)��c(Na��)��c(A)����C����
��Ka����֪�����Һ���ε�ˮ���������ĵ��룬���Ũ�ȵ�NaA��HA�����Һ�У� c(HA)��c(Na��)��c(A)����C����
D������HF��CH3COOH�����������ˮ��̶�F��CH3COO������Һ��c(F)��c(CH3COO)�����ʵ���Ũ����ȵ�NaF��CH3COOK��Һ���������غ��c(Na��)��c(K��)����֪c(Na��)c(F)��c(K��)c(CH3COO)����D����
�ʴ�ѡA��

 �����ܾ�ϵ�д�
�����ܾ�ϵ�д� ���ƿ�����ϵ�д�
���ƿ�����ϵ�д� ���¿쳵����������ϵ�д�
���¿쳵����������ϵ�д�����Ŀ���о�NO2��SO2 ��CO�ȴ�����Ⱦ����Ĵ���������Ҫ���塣
��1��һ�������£���2molNO��2molO2���ں����ܱ������з������·�Ӧ��2NO(g)+O2(g)![]() 2NO2(g)�����и�����˵����Ӧ�ﵽƽ��״̬����_____________��
2NO2(g)�����и�����˵����Ӧ�ﵽƽ��״̬����_____________��
A.��ϵѹǿ���ֲ���
B.���������ɫ���ֲ���
C.NO��O2�����ʵ���֮�ȱ��ֲ���
D.ÿ����1 molO2ͬʱ����2 molNO
��2��CO�����ںϳɼ״���һ���¶��£������Ϊ2L���ܱ������м���CO��H2��������ӦCO(g)+2H2(g)![]() CH3OH(g)����ƽ����ø����Ũ�ȣ�
CH3OH(g)����ƽ����ø����Ũ�ȣ�
���� | CO | H2 | CH3OH |
Ũ�ȣ�mol/L�� | 0.9 | 1.0 | 0.6 |
�ش��������⣺
�ٻ�������ƽ����Է�������=_________________��
��ƽ�ⳣ��K=__________________��
�������������ѹ��Ϊ1L���������㣬Ԥ����ƽ����c(H2)��ȡֵ��Χ��__________��
��������������䣬�ٳ���0.6molCO��0.4molCH3OH����ʱv��______v�������������������=������