��Ŀ����
����Ŀ��������C��H��O���л���3.24 g��װ��Ԫ�ط���װ�ã�ͨ��������O2ʹ����ȫȼ�գ������ɵ���������ͨ���Ȼ��Ƹ����A�ͼ�ʯ�Ҹ����B�����A������������2.16g��B��������9.24g����֪���л������Է�������Ϊ108��
��1��ȼ�մ˻�����3.24g�������������������Ƕ���______��
��2����˻�����ķ���ʽ______����Ҫ�м�����̣�
��3���û�����1�����д���1��������1���ǻ�����д������ͬ���칹��Ľṹ��ʽ______��������Ҫ������˵�����̣�
���𰸡�8.16gC7H8O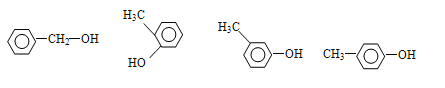
��������
�����ɵ���������ͨ��CaCl2��(A)�ͼ�ʯ��(B),A������������2.16g��ӦΪˮ��������B��������9.24g��ӦΪ������̼�������������л������Է�����������֪��һ�����ʵ������л����к��е�C��Hԭ�Ӹ�������������غ�����Ƿ���O��������Oԭ�Ӹ������Դ˼������ʽ���������غ�ĽǶȼ��������������л���������жϿ��ܵĽṹ��
(1)A������������2.16g Ϊ����ˮ��������B��������9.24gΪ���ɶ�����̼�����������������غ��֪����������������=2.16+9.24-3.24=8.16g����ȷ����8.16g��
(2)�Թ�A��ˮ��������2.16g ,Ϊ����ˮ������,ˮ�����ʵ���=2.16/18=0.12mol,��HΪ0.24mol����ʯ����CO2����9.24g ,�����ɶ�����̼�����ʵ���=9.24/44=0.21mol�����л������ʵ���Ϊ=3.24/108=0.03mol�������л����������N(C)=0.21/0.03=7�� N(H)=0.12��2/0.03=8��N(O)=(108-12��7-8)/16=1�������л���ķ���ʽΪ��C7H8O ����ȷ����C7H8O��
(3)�����л������ڷ����廯����,����1������, C7H8O �IJ����Ͷ�=��2��7+2-8��/2=4���������������ͼ�����ֻ��1����������Ϊ-CH2OH��-OCH3����������2����Ϊ-OH��-CH3,���ڡ��䡢������λ�ù�ϵ���û�����1�����д���1��������1���ǻ����ʷ����������л���Ľṹ��ʽΪ: ����ȷ����
����ȷ���� ��
��

 ����С״Ԫ��������������ϵ�д�
����С״Ԫ��������������ϵ�д�����Ŀ���±�ΪԪ�����ڱ���һ���֣��ش��������⣺
�� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
�� | �� | �� | �� | �� | ||||
�� | �� | �� | �� | �� | ||||
�� | �� | �� |
(1)д��Ԫ�ط��ţ���________����_______��
(2)�ٵ�ԭ�ӽṹʾ��ͼΪ_________���ݵ����ӽṹʾ��ͼΪ________��
(3)Ԫ�آ������������Ӧ��ˮ����ĵ���ʽΪ________��
(4)Ԫ�ܡ��ࡢ����⻯��ķе��ɸߵ��͵�˳��Ϊ_________(�ѧʽ)��
(5)д��Ԫ�آۺ͢��γɵĻ�������Ԫ�آݵ�����������Ӧ��ˮ���ﷴӦ�Ļ�ѧ����ʽ_____________��