��Ŀ����
�����һ����Ҫ�Ŀ�ѧ�о�������ij��ѧʵ��С�����AgOH�����ڰ�ˮ�����ʣ��²�Cu(OH)2Ҳ����ͬ�������ʣ����Դ˽���ʵ��̽����
��1��д��AgOH�백ˮ��Ӧ�����ӷ���ʽ_____________________________________��
��2������ͬѧ�������²����������ྻ���Թ��м���CuSO4��Һ������������μ��백ˮ���۲쵽��ɫ�������ɣ������μӣ��۲������ɴ˵ó�Cu(OH)2�����ڰ�ˮ�Ľ��ۡ�
��д��������ɫ���������ӷ���ʽ��_______________________________________________
��д��������еμӰ�ˮ��ʵ������______________________________________________
��3������ͬѧ��CuSO4��Һ�еμ�NaOH��Һ���Ƶ�Cu(OH)2����ҺA�������м���������ˮ��ʼ��δ�۲쵽��2���е�����������ͬѧ�ڶ����ǵ�ʵ�鼰������бȶԺ�����Cu(OH)2�백ˮ��Ӧ�Ļ�������̽����
�ٸ�ʵ��С���ͬѧȡһ���������Ƶõ�Cu(OH)2����ҺA�������м�����������NH4Cl��Һ������۲쵽��2�������ݴˣ���ͬѧ��Ϊ���ڱ���NH4Cl��Һ�ļ���������Cl������ʹCu(OH)2�백ˮ�ķ�Ӧ���Է�������ָ����ʦ��ʾ��һ�۵��Dz������ġ�Ϊ���ų�Cl���ĸ��ţ�����ͬѧ����������ʵ�飺ȡһ���������Ƶõ�Cu(OH)2����ҺA�������м��뱥��___________��Һ���ѧʽ�������ͬ���۲쵽��2���е����ݴ˿�֪�÷�Ӧ�ķ�����Cl���ء�
����ˣ�ͬѧ���������Cu(OH)2�백ˮ�ķ�Ӧ�ķ�����ҪNH4+�IJ��룬����ͬѧ�ǽ�һ��̽���������ྻ���Թ��м���CuSO4��Һ������������μ��백ˮ���۲쵽��ɫ�������ɡ�ͨ�����ĵķ����õ�Cu(OH)2������������ϴ�Ӻ����ڸ���ྻ���Թ��У����Թ��еμ��������Ͱ�ˮ��Ϊ�۲쵽��2�������������Թ��еμ���������NH4Cl��Һ���۲쵽��2��������
��i�������Ƿ����ϴ�Ӹɾ��IJ�����___________________________________________
��ii���ݴˣ����Եó����ۣ���Cu(OH)2�백ˮ�ķ�Ӧ�У�NH4+����������Ҫ�����ã���________________�Է�Ӧ�ķ�����Ӱ�졣
��4���Դ��ܽ�ƽ��ǶȽ���������Ӧ����������NH4+�����ã�___________________________��
��1��AgOH+2NH3��H2O=Ag��NH3��2+ +OH- +2H2O
��2����Cu2++2NH3��H2O=Cu(OH)2��+2NH4+ ����ɫ�������ܽ�
��3���٣�NH4��2SO4
�ڣ�i��ȡ���һ��ϴ�Ӻ��ϴ��Һ�������м���BaCl2��Һ�������������������ϴ������������ɫ��������δϴ�Ӹɾ���
(ii)笠����ӵ�Ũ��
��4������Һ���д���笠����Ӵ���ʱ��笠�����������������ѵ����һˮ�ϰ���ʹ��Һ������������Ũ�Ƚ��ͣ�����ƽ�������ƶ�����ɫ��������ͭ�������ܽ⡣
���������������1����AgOH�еμӰ�ˮ����������������ӡ�
��2������ɫ����Ϊ������ͭ������ɫ�������ܽ⣬������ɵ�ͭ��������ӡ�
��3����Ϊ�ų��������ӵĸ��ţ��ɼ��루NH4��2SO4��
�ڣ�i���ó����п��ܺ��е��������������������˼�����������ɡ�
��ii����ʵ����������õ��DZ�����Һ�����NH4+��Ũ�ȶԷ�ӦҲ��Ӱ�졣
��4��ʵ������д��ڵ�ƽ���У�Cu(OH)2 Cu2++2OH����OH��+NH4+
Cu2++2OH����OH��+NH4+ NH3��H2O������Һ���д���笠����Ӵ���ʱ��笠�����������������ѵ����һˮ�ϰ���ʹ��Һ������������Ũ�Ƚ��ͣ���һ��ƽ�������ƶ�����ɫ��������ͭ�������ܽ⡣
NH3��H2O������Һ���д���笠����Ӵ���ʱ��笠�����������������ѵ����һˮ�ϰ���ʹ��Һ������������Ũ�Ƚ��ͣ���һ��ƽ�������ƶ�����ɫ��������ͭ�������ܽ⡣
���㣺�������ӷ���ʽ��д����ѧʵ��ԭ����ʵ����������������ܽ�ƽ��ȡ�

 ��ʦ������Ԫ��ĩ���100��ϵ�д�
��ʦ������Ԫ��ĩ���100��ϵ�д� ��У������Ԫͬ��ѵ��������ϵ�д�
��У������Ԫͬ��ѵ��������ϵ�д���������п[(C6H11O6O)2Zn]��һ��Ӫ��пǿ��������Ӥ��������������������巢������Ҫ���á���ҵ��ͨ�����������Ʊ���
��1������һ����ַ�Ӧ���˳�ȥCaSO4���������һ����ʵ�飬�����������������Һ���Ƿ���SO42���� ��
��2���������������������Һ��ZnO��ϣ�ʹ���ַ�Ӧ������������������Һ��pHΪ5.8����Ŀ���� ���������ʿ����ZnO���� (����ĸ)��
a��NH3��H2O b��Zn(OH)2 c��NaOH d��ZnSO4
��3�������������ҺŨ����ԭ������� ������������ˮ�Ҵ�������8h���ϣ����ᾧ�����롢��������������п���塣��������м�����ˮ�Ҵ���Ŀ���� ��
������������ˮ�Ҵ�������8h���ϣ����ᾧ�����롢��������������п���塣��������м�����ˮ�Ҵ���Ŀ���� ��
��4���±��г�����ؽ������������������������pH(��ʼ������pH����������Ũ��Ϊ1.0 mol��L��1����)��
| �������� | ��ʼ������pH | ������ȫ��pH |
| Fe3�� | 1. 1 | 3. 2 |
| Fe2�� | 5. 8 | 8. 8 |
| Cu2�� | 5. 2 | 6. 4 |
ij�о���ѧϰС�����ô�������п��Һ�����к���Fe2+��Cu2+�ȣ��Ʊ�������ZnO��Ȼ���ٺϳ���������п��ʵ�����Ʊ�����ZnO�IJ������£�
��ȡ��������������KMnO4��Һ���ȣ�������ҺpH�� ����д��Χ������ȥ��Һ��FeԪ�ء�
�ڼ��� �����ˣ��������м�������ϡ���ᣬ�������ˣ���������Һ�ϲ��ýϸߴ��ȵ�����п��Һ��
�۽���������������������п��Һ�У��ü�ʽ̼��п[�仯ѧʽΪZn2(OH)2CO3]��ͬʱ����ɫ���������д���÷�Ӧ�����ӷ���ʽ�� ��
�ܹ��ˡ�ϴ�ӣ����������յû�������п������������Ҫ����Ҫ�����У��ƾ��ơ������������żܡ������ǡ� �ȡ�
��0.1320 mol/L��HCl��Һ�ζ�δ֪Ũ�ȵ�NaOH��Һ��ʵ���������±���ʾ��
| ʵ���� | ����NaOH��Һ�����/mL | HCl��Һ�����/mL |
| 1 | 25.00 | 24.41 |
| 2 | 25.00 | 24.39 |
| 3 | 25.00 | 22.60 |
��1��ͼ�м�Ϊ �ζ��ܣ���Ϊ �ζ��ܣ����ʽ����ʽ ������

��2��ȡ����ҺNaOH��Һ25.00 mL ����ƿ�У�ʹ�� ��ָʾ�����ζ��յ���ж������� ��
��3�����ζ�ǰ���ζ��ܼ�������ݣ��ζ���������ʧ����ʹ������ ���ƫ�ߡ�����ƫ�͡����䡱����ͬ����������ʽ�ζ��ܶ���ʱ���ζ�ǰ���Ӷ������ζ�����ȷ�������������� ��
��4����NaOH��Һ�����ʵ���Ũ��Ϊ mol/L (����С�������λ��Ч����)��
ijѧ������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�NaOH��Һ��
������ɷֽ�Ϊ���¼�����
a. ��ȡ20.00mL�����NaOH��Һע��ྻ����ƿ��������2-3�η�̪
b. �ñ�������Һ��ϴ�ζ���2-3��
c. ��ʢ�б���Һ����ʽ�ζ��̶ܹ��ã�����Һ��ʹ�ζ��ܼ��������Һ
d. ȡ��������Һע����ʽ�ζ�����0�̶�����2-3cm
e. ����Һ����0��0�̶����£����¶���
f. ����ƿ���ڵζ��ܵ����棬�ñ�������Һ�ζ����յ㣬���µζ���Һ��Ŀ̶�
���������գ�
��1����ȷ������˳���ǣ��������ĸ��д��__________________ ____��
��2���ζ��յ�ʱ��Һ����ɫ�仯�� ��
��3�����в����п���ʹ����NaOH��Һ��Ũ����ֵƫ�͵���_____ ___��
| A����ʽ�ζ���δ�ñ�������ϴ��ֱ��ע������� |
| B���ζ�ǰʢ��NaOH��Һ����ƿ������ˮϴ����û�и��� |
| C����ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ |
| D����ȡ�������ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ��� |
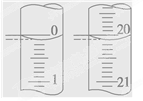
��5��ijѧ������3��ʵ��ֱ��¼�й��������±���
| �ζ� ���� | ����NaOH��Һ�����/mL | 0.1000mol/L��������/mL | ||
| �ζ�ǰ�̶� | �ζ���̶� | ��Һ���/mL | ||
| ��һ�� | 25.00 | 0.20 | 20.22 | |
| �ڶ��� | 25.00 | 0.56 | 24.54 | |
| ������ | 25.00 | 0.42 | 20.40 | |
�����ϱ��������NaOH��Һ�����ʵ���Ũ��Ϊ ��
���ⶨijNaOH��Һ�����ʵ���Ũ�ȣ�����0.1000mol��L-1HCl����Һ�����к͵ζ����ü�����ָʾ������
��ش��������⣺
��1���ζ�ʱ��ʢװ����NaOH��Һ����������Ϊ ��ʢװ���������������Ϊ ���ζ����յ����ɫ�仯Ϊ ��
��2������ѧ����ʵ������У���¼�ζ�ǰ�ζ�����Һ�����Ϊ0.50mL���ζ���Һ����ͼ�����ʱ���ı���Һ�����Ϊ ��
��3����ѧ����������ƽ��ʵ�飬���ݼ�¼���£�
| ʵ����� | ����NaOH��Һ�����/mL | 0.1000mol��L-1HCl��Һ�����/mL | |
| �ζ�ǰ�̶� | �ζ���̶� | ||
| 1 | 25.00 | 0.00 | 26.29 |
| 2 | 25.00 | 1.56 | 31.30 |
| 3 | 25.00 | 1.00 | 27.31 |
ѡȡ�����������ݣ����������NaOH��Һ�����ʵ���Ũ��Ϊ ��������λ��Ч���֣���
��4��������Щ������ʹ�ⶨ���ƫ�� ������ţ���
A.��ƿ������ˮϴ�������ô���Һ��ϴ
B.��ʽ�ζ���������ˮϴ�������ñ�Һ��ϴ
C.�ζ�ǰ��ʽ�ζ��ܼ������δ�ų����ζ���������ʧ
D.�ζ�ǰ������ȷ���ζ����ӵζ��ܶ���
����������ijͬѧ����ͭпԭ���ʵ���ó��Ľ��ۺ���ʶ,��ȷ����(����)
| A������ԭ������������IJ��ϱ��������ֽ��� |
| B����ͭп��Ϊ�缫������ͭ��Һ��ɵ�ԭ�����,ͭ�Ǹ��� |
| C������ͨ��������Һ��SO42- �������ƶ� |
| D��ͭпԭ��ع���ʱ,����13 gп���ܽ�,��·�о���0.4 mol ����ͨ�� |
��ʯī�缫���CuCl2��Һ������ͼ�������з�����ȷ����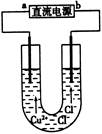
| A��a����ֱ����Դ�ĸ��� |
| B��ͨ��ʹCuCl2�������� |
| C�������Ϸ����ķ�Ӧ��Cu2++2e��=Cu |
| D��ͨ��һ��ʱ��������������۲쵽����ɫ���� |
������ȼ�ϵ�ؾ��иߵķ���Ч�ʣ�����ܵ����ӡ���Li2CO3��Na2CO3�������λ����������ʣ�һ��ͨCO���壬��һ��ͨO2��CO2������壬���Ƶ���650���¹�����ȼ�ϵ�ء���֪�õ���ܷ�ӦΪ��2CO+O2��2CO2��������˵������ȷ����( )
| A��ͨCO��һ���ǵ�ص����� �� |
| B���õ�ع����������費�ϲ���CO��O2��CO2��ѭ������ |
| C��������ӦʽΪ��O2+2CO2+4e��==2CO32�� |
| D��������ӦʽΪ��2CO+2CO32����4e��==4CO2 |

 LaNi5+6Ni(OH)2���õ�طŵ�ʱ��������Ӧ�� ��
LaNi5+6Ni(OH)2���õ�طŵ�ʱ��������Ӧ�� ��