��Ŀ����
3�������⡢���������ơ��ơ������ȡ�ͭ8��Ԫ�أ���1��ͭλ�����ڱ� ���ڵ������ڢ�B�壮
��2���á���������������д�±�
| ��һ������ | �縺�� | ������ | �е� |
| O��N | Cl��F | NaCl��CaO | HF��HCl |
| ���ۼ� | H-Cl | O=O | H-O | Cl-Cl |
| ����/kJ•mol-1 | 431 | 498 | 463 | 243 |
��4��ͭ����Ũ���Ỻ�������û���Ӧ���������H[CuCl2]���ɣ�
�ٸ÷�Ӧ�Ļ�ѧ����ʽΪ2Cu+4HCl��Ũ��=2H[CuCl2]+H2����
��H[CuCl2]�ڿ����о��û����ɺ�[Cu��H2O��4]2+����ɫ��Һ��[Cu��H2O��4]2+�Ľṹ����ʾ��ͼ��ʾΪ
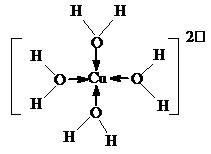 ��
��
���� ��1��ͭ��29�ţ�λ�����ڱ����������ڢ�B�壻
��2��ͬ���ڴ����ҵ�һ�����������ڢ�A�͵ڢ��������Ԫ�شǽ�����Խǿ�縺��Խ��������Խ�࣬�뾶ԽС������Խ������������ʷе�ϸߣ�
��3����д����ѧ����ʽ��Ȼ������ʱ�=��Ӧ����ܼ���-��������ܼ��ܼ����ʱ䣻���Ȼ�ѧ����ʽ��������H��ֵ��
��4����ͭ����Ũ���Ỻ�������û���Ӧ���������H[CuCl2]���ɣ����Է�Ӧ����ʽΪ��2Cu+4HCl��Ũ��=2H[CuCl2]+H2����
��[Cu��H2O��4]2+��ͭ�����ṩ�չ������ԭ���ṩ���Ӷ��γ���λ����
��� �⣺��1��ͭ��29�ţ�λ�����ڱ����������ڢ�B�壬�ʴ�Ϊ���������ڢ�B��
��2��ͬ���ڴ����ҵ�һ�����������ڢ�A�͵ڢ��������Ԫ�ش�����O��һ������С��N��һ�����ܣ��ǽ�����F����Cl�����Ե縺��F����Cl����������Խ�࣬�뾶ԽС������Խ�������Ȼ��Ƶľ�����С�������ƣ���������Ӽ������������Էе�HF����HCl���ʴ�Ϊ������������������
��3��������Ӧ�Ļ�ѧ����ʽΪO2��g��+4HCl��g��=2Cl2��g��+2H2O��g������H=��Ӧ����ܺ�-��������ܺ�=498KJ/mol+4��431KJ/mol-2��243KL/mol-4��463KJ/mol=-116KJ/mol�����ԣ����Ȼ�ѧ����ʽΪ��O2��g��+4HCl��g��=2Cl2��g��+2H2O��g����H=-116KJ/mol���ʴ�Ϊ��O2��g��+4HCl��g��=2Cl2��g��+2H2O��g����H=-116KJ/mol��
��4����ͭ����Ũ���Ỻ�������û���Ӧ���������H[CuCl2]���ɣ����Է�Ӧ����ʽΪ��2Cu+4HCl��Ũ��=2H[CuCl2]+H2�����ʴ�Ϊ��2Cu+4HCl��Ũ��=2H[CuCl2]+H2������[Cu��H2O��4]2+��ͭ�����ṩ�չ������ԭ���ṩ���Ӷ��γ���λ��������[Cu��H2O��4]2+�Ľṹ����ʾ��ͼ��ʾΪ

���� ���⿼���˻�ѧ��Ӧ�������仯���һ�����ܡ��縺�ԡ������ܡ��е��Լ���ѧ����ʽ����д���ۺ���ǿ�����Ƚϻ�����

 ���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
���ɿ��õ�Ԫ������ĩר����100��ϵ�д�| A�� | CH3CH2OH+CH3COOH$��_{��}^{����}$ CH3COOCH2CH3+H2O | |
| B�� | 2CH3CHO+O2 $��_{��}^{����}$2CH3COOH | |
| C�� | CH3-CH=CH2+Br2��CH3-CHBr-CH2Br | |
| D�� |  +Br2 $\stackrel{Fe}{��}$ +Br2 $\stackrel{Fe}{��}$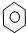 -Br+HBr -Br+HBr |
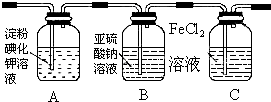
��1��ͨ��������A�е���������Һ����ɫ��Cװ���з��������ӷ���ʽΪ2Fe2++Cl2�T2Fe3++2Cl-��
��2��ͨ������һ��ʱ���ϴ��ƿB��Һ����һ����SO32-������SO42-��������鷽��������ϴ��ƿB��Һ��Cl-��SO42-�Ĵ��ڣ���д��ʵ�鲽�衢Ԥ������ͽ��ۣ���������Ҳ�ɲ�������
��ѡ�Լ���������2mol/L HCl��2mol/L HNO3��1mol/L BaCl2��Һ��l mol/L Ba��NO3��2��Һ��0.1mol/L AgNO3��Һ������ʯ��ˮ���Թܡ���ͷ�ιܣ�
| ʵ�鲽�� | Ԥ������ͽ��� |
| ����1��ȡ����ϴ��ƿB����Һ���Թ�A�У��μӹ���2 mol/LHCl��1 mol/LBaCl2��Һ���� | �������İ�ɫ��������ϴ��ƿB��Һ�д���SO42-�� |
| ����2����ȡ����ϴ��ƿB����Һ���Թ�B�У��μӹ���l mol/LBa��NO3��2��Һ�������ã� | ������ɫ������ |
| ����3��ȡ����2���Թ�B�е��ϲ���Һ���Թ�C�У��μ�0.1mol/LAgNO3��Һ������2mol/LHNO3���� | ��������ɫ��������ϴ��ƿB��Һ�д���Cl- |
| ѡ�� | ����ʽ | ����ԭ���ӻ���ʽ | �۲���ӶԻ���ģ�� | ���ӻ��������幹�� |
| A | SO2 | sp | ֱ���� | ֱ���� |
| B | HCHO | sp2 | ƽ�������� | ������ |
| C | NF3 | sp2 | �������� | ƽ�������� |
| D | NH4+ | sp2 | ���������� | ���������� |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | ����Ǧ���طŵ�ʱ�������������ᣬ������������ | |
| B�� | ��ⷨ����ͭʱ���Դ�ͭ����������ͭ������ | |
| C�� | �����£�pH=5������ϡ��1000������Һ��pH=8 | |
| D�� | ˮ�����ӻ����¶����߶�����˵��ˮ�ĵ��������ȷ�Ӧ |
| A | B | C | D |
 | 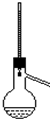 | 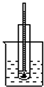 | 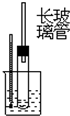 |
| A�� | �ƾ���Ũ�����ϼ�������ϩ | B�� | ʯ�ͷ���ʵ�� | ||
| C�� | �������ȡ����Ӧ | D�� | ��ʯ��ˮ��Ӧ����Ȳ |
| A�� | HAt���ȶ� | B�� | At����ɫ���� | ||
| C�� | At�������л��ܼ� | D�� | AgAt������ˮ |
| ʱ��/min | 0 | 1 | 2 | 3 | 4 | 5 |
| �������/mL | 0 | 50 | 120 | 232 | 290 | 310 |
��2����2��3minʱ����������Ũ�ȱ仯����ʾ�ĸ÷�Ӧ���ʣ�����Һ������䣩0.1mol/��L��min����
��3��Ϊ�˼�����Ӧ���ʶ��ֲ����ٲ����������������������м���������Һ�е�AD�����ţ���
A������ˮ B��NaNO3��Һ C��Na2CO3��Һ D��CH3COONa��Һ E��CuSO4��Һ��
| A�� | ���淴Ӧ�ﵽ��ѧƽ��״̬ʱ�������淴Ӧ���ʾ������� | |
| B�� | ����Ӧ����������Ũ�����ʱ�����淴Ӧһ���Ѵﵽ��ѧƽ��״̬ | |
| C�� | ��п�۴���п�����Ũ�ȵ�ϡ���ᷴӦ���������������ʼӿ� | |
| D�� | Fe��ϡ���ᷴӦ��ȡ����ʱ������Ũ�����������Ӧ���� |