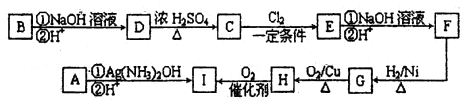
��Ŀ����
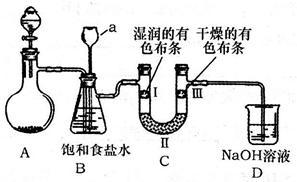
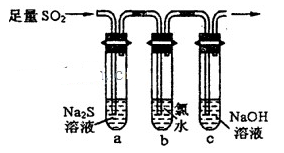
ijУ������ѧ�о���ѧϰС�飬��ѧϰ��ͭ��Ũ����ķ�Ӧ��ֱ�̽��������п��Ũ���ᷴӦ�Ĺ��̡���С���������ͼװ�ã��Իش�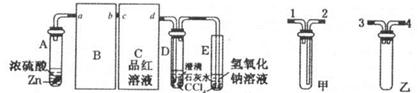
(1)��Ӽס�����ѡ����ʵ�װ������B��C�У���������ȷ���ӣ�a��______, c��______(����ű�ʾ����
(2)D��E��֧�Թ���CCl4��������______��
(3)��ʵ����֤��Ũ�������ǿ�����Ե�ʵ������Ϊ______��
(4)D�г��ֻ��ǵ����ӷ���ʽΪ______��
(5)ijѧ��ע��۲쵽��ʵ�鿪ʼ��C��D��E�о������ݲ�����������������٣�Ʒ����Һ��ɫ��D�г��ֻ��ǣ���Ӧһ��ʱ���C��D��E�е��������ֻ��������ӡ����û�ѧ����ʽ��ʾ����Ӧһ��ʱ����������ֻ��������ӡ���ԭ����______��
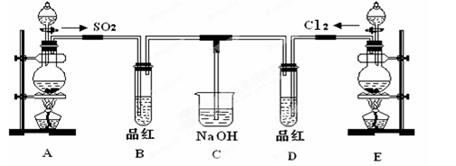
��С���������ͼװ�ã�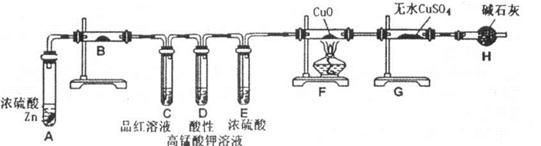
(6)��������С��������ͼ��______��
(7)װ��B�е�ҩƷ��______��
(8)��֤������SO2��H2�Ⱥ�˳���ʵ��������______��
��15�֣�(1) 3(��4) 2����1�֣���2�֣� (2) ��������2�֣�
(3) C��Ʒ����Һ��ɫ��2�֣�
(4) Ca2+��2OH-��SO2===CaSO3��+H2O��2�֣�
(5) Zn+H2SO4��ϡ���� ZnSO4+H2����2�֣�
(6) ��֤�������Ȳ���ˮ��SO2�������H2��2�֣����������𰸲��ո��֣�
(7) ��ˮCuSO4(����ˮ����ͭ) ��1�֣�
(8) C��Ʒ����ɫ��D�����Ը��������ɫ���ٱ�dz��F�й����ɺ�ɫ���ɫ��G����ˮ����ͭ������2�֣����������𰸲��ո��֣�
���������������1������ͭ��Ũ���ᷴӦ�ķ���ʽ��֪��п��Ũ���ᷴӦ�ķ���ʽΪZn+2H2SO4��Ũ���TZnSO4+SO2��+2H2O������ͨ��״���£�1���ˮ�п��ܽ�40����Ķ����������壬���װ���ҿɷ�ֹ����������Ʒ������ʱ������п�����ᷴӦ�������У�����������Ʒ�����ã��賤�ܽ���������ȷ������˳����3����4����4����3����b��c��2��1��d��
��2����������������ˮ�����������Ȼ�̼����������ͨ�����Ȼ�̼��Ȼ����������ʯ��ˮ��Ӧ�������ã��ɷ�ֹ������
��3�����������ʹƷ����Һ��ɫ�������ڼ����������Ĵ��ڣ����Ա�ʵ����֤��Ũ�������ǿ�����Ե�ʵ������ΪC��Ʒ����Һ��ɫ��
��4����������ͳ����ʯ��ˮ��Ӧ����������ƺ�ˮ���������Ϊ������ˮ�ij�������Ӧ�����ӷ���ʽΪCa2++2OH-+SO2�TCaSO3��+H2O��
��5��пΪ�ϻ��õĽ������ڽ����˳������������ǰ�棬����п�ܺ�ϡ���ᷴӦ��������п�������������ŷ�Ӧ�Ľ��У�Ũ�����Ũ�����ͣ�����Ϊϡ�������п��Ӧ������������Ӧ�Ļ�ѧ����ʽΪZn+H2SO4��ϡ���TZnSO4+H2����
��6������װ��ͼ��֪����С����Ƶ�װ�ã����ð�ɫ����ˮ����ͭ����ˮ�Ĵ��ڣ�����Ʒ������������Ĵ��ڣ��ø�������������������ȥ��������Ũ���������ˮ�ԣ���ȥˮ��п��ϡ���ᷴӦ��������������п������ͨ���ȵ�����ͭ����ͭ��ˮ��ˮ��������ˮ����ͭ����ɫ���ɼ��������Ĵ��ڣ���˸���Ƶ�Ŀ������֤�������Ȳ���ˮ��SO2�������H2��
��7����������ͨ����Һʱһ�������ˮ����������Ҫ���ȼ���ˮ����������ˮ��������ˮ����ͭ����ɫ��֪��װ��B�е�ҩƷ����ˮCuSO4������ˮ����ͭ������������ˮ�Ĵ��ڡ�
��8�����������ʹƷ����Һ��ɫ��C��Ʒ����ɫ���ɼ����������Ĵ��ڡ�SO2�����л�ԭ�ԣ��������������������ɳ�ȥ��������D�����Ը�����ز�����ɫ��˵�����������Ѿ�������п��ϡ���ᷴӦ��������������п������ͨ���ȵ�����ͭ����ͭ��ˮ��F���к�ɫ�������ɣ�˵����ͭ���ɣ�G����ˮ����ͭ������˵����ˮ���ɣ���֤��������������
���㣺����п�����ᷴӦ�����������й��жϡ�ʵ����������Լ�ʵ�鷽����������۵�

 ����ѧ���ʱѧ����ϵ�д�
����ѧ���ʱѧ����ϵ�д� �ʰ�Ӣ��ͬ����ϰ��ϵ�д�
�ʰ�Ӣ��ͬ����ϰ��ϵ�д���Ϥʵ������������ȷ����ʵ����������û�ѧʵ���ǰ�ᡣ
��1�������й�ʵ�������ʵ����ʵ����������ȷ���� (�����)��
A��ʵ������Ũ����Ӧ��������ɫϸ��ƿ�У���������ͼ��ʾ��ǩ |
| B����50mL��Ͳ��ȡ5��6mLŨ���� |
| C���к͵ζ�ʵ��ʱ����ƿϴ�Ӹɾ����ñ�Һ��ϴ����ע�����Һ |
| D�������Ȼ�̼��ȡ��ˮ�еĵ⣬��Һʱ�л���ӷ�Һ©�����¶˷ų� |
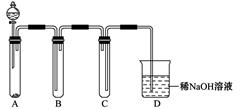
��2����ͼ��ʵ�����Ʊ�������̽�������Ƿ����Ư���Ե�ʵ��װ��(�гּ�����������ʡ��)��
��Aװ���з�Ӧ�Ļ�ѧ����ʽΪ ��
��Bװ��������a�������� ��
��Bװ�õ������dz�ȥ�����л��е�HCl������ȫƿ�����ã�������a��Һ�治
������ʱ��˵�� ����ʱӦֹͣʵ�顣
��ʵ���й۲쵽 ��˵������������Ư���ԡ�
�ף��������о���ѧϰС��Ϊ�ⶨ�������е�����ԭ�Ӹ����ȣ����������ʵ�����̣�
��ͼA��B��CΪ�ף�����С����ȡ����ʱ�����õ���װ�ã�DΪʢ��Ũ�����ϴ��ƿ��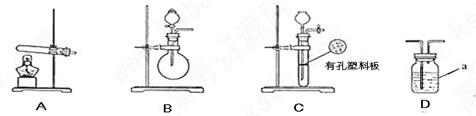
ʵ�鿪ʼǰװ���еĿ������ž�����С���ã���Ӧǰ����ͭ������Ϊ ������ͭ��Ӧ��ʣ����������Ϊ
������ͭ��Ӧ��ʣ����������Ϊ �����ɵ����ڱ�״���µ����
�����ɵ����ڱ�״���µ���� ����С����ϴ��װ��Dǰ������������ɵ����ڱ�״���µ������
����С����ϴ��װ��Dǰ������������ɵ����ڱ�״���µ������
��1��д������a�����ƣ� ��
��2���ף�����С��ѡ���˲�ͬ������ȡ�������뽫ʵ��װ�õ���ĸ��ź��Ʊ�ԭ����д���±��ո��С�
| | ʵ��װ�� | ʵ��ҩƷ | �Ʊ�ԭ�� |
| ��С�� | A | �������ƣ������ | ��Ӧ�Ļ�ѧ����ʽΪ �� |
| ��С�� | �� | Ũ��ˮ���������� | �û�ѧƽ��ԭ�������������Ƶ����ã� �� |
��3����С�����������ݼ�����������е������ԭ�Ӹ���֮��Ϊ ��
��4���ڲ����ͼ�����ȷ������£���С�����������ݼ�����������е������ԭ�Ӹ���������С������ֵ����ԭ������� ��
��5����С����ԭ��ʵ��Ļ�����������һ��װ��ҩƷ��ʵ������������ʵ�飬�ó�������ʵ��������ҩƷ�������� ��
���ü�������������ȡ����Ӧ��ȡ����Ʒ����������ڹ�ҵ���ѳ�Ϊ��ʵ��ij��ѧ��ȤС��ͨ����ʵ������ģ���������̣�����Ƶ�ģ��װ�����£�
��1�� Bװ�������ֹ��ܣ��ٿ��������ٶȣ��ھ��Ȼ�����壻��
|
��4�� ��Cװ���У�����һ��ʱ���ǿ�����䣬����Ӳ�ʲ������ڱ��к�ɫС����������д���û�����ɫС�����Ļ�ѧ����ʽ ��
��5�� Eװ���г������⣬�������л����E�з�����������ѷ���Ϊ ��
A.��Һ�� B.���� C.��ȡ��Һ�� D.�ᾧ��
��6����װ�û���ȱ�ݣ�ԭ����û�н���β����������β����Ҫ�ɷ�Ϊ �����ţ�
A��CH4 B��CH3Cl C��CH2Cl2 D��CHCl3

 ���÷�Ӧ�б�������Ԫ����________����Ԫ�ط��ţ���
���÷�Ӧ�б�������Ԫ����________����Ԫ�ط��ţ���