��Ŀ����
I��������������Ҫ�Ĺ�ҵԭ�ϣ�̽�����Ʊ����������ʾ��зdz���Ҫ�����塣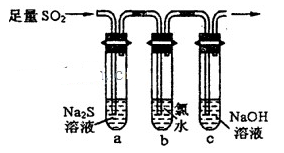
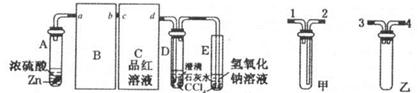
��1����ҵ���û�����FeS2������SԪ��Ϊ��l�ۣ��ڸ����º�������Ӧ�Ʊ�SO2�� ���÷�Ӧ�б�������Ԫ����________����Ԫ�ط��ţ���
���÷�Ӧ�б�������Ԫ����________����Ԫ�ط��ţ���
��2��һ��ѧ�о���ѧϰС�����������װ����֤��������Ļ�ѧ���ʣ�
����˵������������������Ե�ʵ������Ϊ________________________________��
��Ϊ��֤��������Ļ�ԭ�ԣ���ַ�Ӧ��ȡ�Թ�b�е���Һ�ֳ����ݣ��ֱ��������ʵ�飺
���������һ����Һ�м���AgNO3��Һ���а�ɫ��������
��������ڶ�����Һ����Ʒ����Һ����ɫ��ȥ
���������������Һ����BaC!����Һ��������ɫ�������������������Ƿ���______������������Թ�b�з�����Ӧ�����ӷ�Ӧ����ʽΪ____________________��
�۵�ͨ������������Թ�c����Һ������ʱ����Һ��c��Na+����________________���ú���Ԫ����Ũ�ȵĴ���ʽ��ʾ����
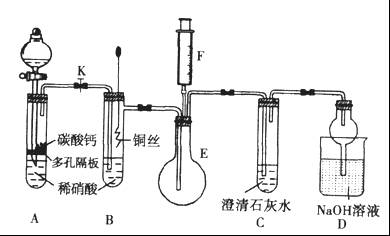
����һ��ѧ�о���ѧϰС����ʵ����������������ͭ��ҺΪ���Һ���õ��ķ���ʵ���˴�ͭ���ᴿ������������͵��Һ�н������л��պͺ����ⶨ����֪��ͭ�к���������п������������Ƚ����������������ʣ������Ӧ����
����һ����⾫�ƣ�
���ʱ����ͭӦ���Դ��______�������������ϵĵ缫��ӦʽΪ____________��
������������ɺ�С��ͬѧ���������̶Ե��Һ���д�����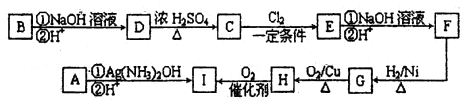
ϡ���ᴦ��������õ�������ϡ��Һ����д���÷�Ӧ�����ӷ���ʽ��
____________________________________________________________��
��1��Fe S
��2�����Թ�a�г��ֵ���ɫ���� �ڢ� SO2 + Cl2 +2H2O = 4H+ + SO42- + 2Cl- (3��)
��2c��SO32-��+ c��HSO3-��
����һ�� �� Cu2+ + 2e- = Cu
������� 3Ag + 4H+ + NO3- = 3Ag+ + NO��+2H2O (3��)
�������������
I����1��������ԭ��Ӧ4FeS2+11O2=8SO2+2Fe2O3�У����ϼ����ߵ�Fe��SԪ���ڷ�Ӧ�б�������
��2���ٶ���������������ԣ����Ժ���ͼ۵����Ʒ���������ԭ��Ӧ�����ɵ���ɫ�ij���S��
�ڷ���I�����һ����Һ�м���AgNO3��Һ���а�ɫ�������ɣ���������ˮ�������Ӳ��������ã�
��������ڶ�����Һ����Ʒ����Һ����ɫ��ȥ����������ˮ�к��е�Ư�������ʴ�����������ã�
���������������Һ����BaCl2��Һ��������ɫ������֤����Һ���ܳ�����������ӣ��Ƕ������������Ի����º���ˮ��Ӧ���ɵģ���SO2+Cl2+2H2O=4H++SO42-+2Cl-��
�۽���������ͨ�뵽ʢ���������Ƶ��Թ�c�У�������Һ�д��ڵ���غ㣺
c��Na+��+c��H+��=2c��SO32-��+c��HSO3-��+c��OH-������Һ��ʾ���ԣ����ԣ�
c��H+��=c��OH-������ʱ��c��Na+��=2c��SO32-��+c��HSO3-����
����һ����ͭӦ�����Դ�������������ô�ͭʧ���ӡ�
�������ϡ����������Ӧ������һ��������
���㣺���⿼���˶�����������Ӧ�ú�ʵ����֤��ʵ����ƵIJ����ʵ�鷽����ȷ��Ӧ�������Ҫ������жϣ��ؼ��Ƕ��������Ϣ������������������жϣ���Ŀ���ѡ�

 8SO2��2Fe2O3�÷�Ӧ�б�������Ԫ����_______����Ԫ�ط��ţ������÷�Ӧת��2. 75mol����ʱ�����ɵĶ��������ڱ�״���µ����Ϊ_______L��
8SO2��2Fe2O3�÷�Ӧ�б�������Ԫ����_______����Ԫ�ط��ţ������÷�Ӧת��2. 75mol����ʱ�����ɵĶ��������ڱ�״���µ����Ϊ_______L��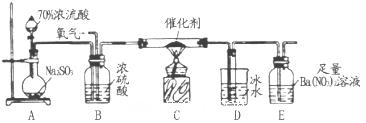
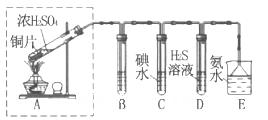
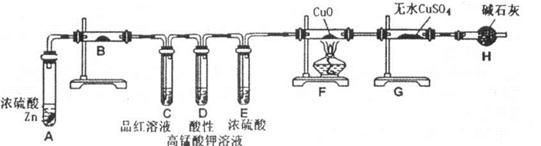
 �Ʊ���ˮ�Ȼ������������£�
�Ʊ���ˮ�Ȼ������������£�
 �ķ�����________________________________��
�ķ�����________________________________��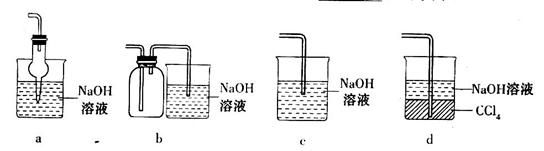
 �ȴ�����Ⱦ��������߰���һ������ͨ��ˮ�пɼ��ٻ�������Ⱦ������Ƽ�ʵ���������Ƿ�ǡ����ȫ��Ӧ������Ҫ����ʵ�鲽�衢����ͽ��ۣ�
�ȴ�����Ⱦ��������߰���һ������ͨ��ˮ�пɼ��ٻ�������Ⱦ������Ƽ�ʵ���������Ƿ�ǡ����ȫ��Ӧ������Ҫ����ʵ�鲽�衢����ͽ��ۣ�
 ��Һ��������________________��
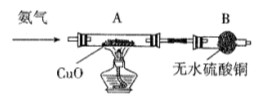
��Һ��������________________�� 2CuO��CuO��H2SO4��CuSO4��H2O��
2CuO��CuO��H2SO4��CuSO4��H2O��