��Ŀ����
�Ȼ�����Ʒ�к�������̼��ء�����غͲ�����ˮ�����ʡ�Ϊ���ᴿ�Ȼ��أ��Ƚ���Ʒ��������ˮ�У���ֽ������ˣ��ٽ���Һ����ͼ��ʾ������в�����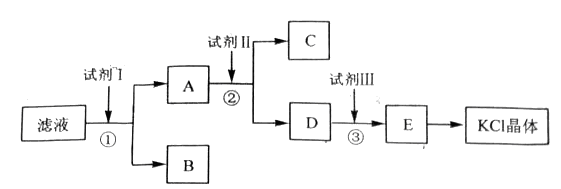
�ش��������⣺
��1����ʼ��Һ��pH_____7(����ڡ���С�ڡ����ڡ�)����ԭ����______________________________��(�����ӷ���ʽ��ʾ)
��2���Լ���Ļ�ѧʽΪ___________�����з�����Ӧ�����ӷ���ʽΪ_______________��
��3���Լ���Ļ�ѧʽΪ____________�����м����Լ����Ŀ����__________________��
��4���Լ����������_____________�����з�����Ӧ�����ӷ���ʽΪ________________��
��1������CO32-+H2O HCO3- +OH- (HCO3-+H2O
HCO3- +OH- (HCO3-+H2O H2CO3+ OH-δд���۷�)
H2CO3+ OH-δд���۷�)
��2��BaCl2 Ba2++SO42-=BaSO4 ��Ba2++CO32��=BaCO3��д���۷֣�
��3��K2CO3 ��ȥ������Ba2+
��4������ CO32�� +2H+=H2O+CO2
���������������1����ʼ��Һ�к���̼��أ�̼���ˮ��ʼ��ԣ�����Һ��PH����7����2��Ҫ�������������������̼�����Ӧ��������ı����ӣ���3��Ҫ��������ı����ӣ�Ҫ����̼��أ���4��Ҫ���������̼�����Ҫ�μ����������
���㣺��Һ������Ե�ԭ���ӵķ��������衢���ӷ���ʽ����д��

 ��������һ���þ�ϵ�д�
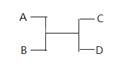
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
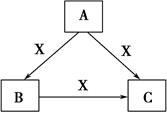
Сѧ��10����Ӧ����ϵ�д��ס��ҡ����������������У��ס��ҡ�����������ͬ��ij��Ԫ�أ�����֮���������ת����ϵ��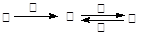 �������й����ʵ��ƶϲ���ȷ����
�������й����ʵ��ƶϲ���ȷ����
| A������Ϊ��̿��������O2 |
| B������ΪSO2�������ǰ�ˮ |
| C������ΪFe������������ |
| D������ΪNaOH��Һ��������CO2 |

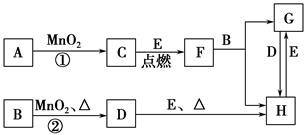
 Fe2O3 + 2Al
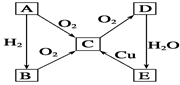
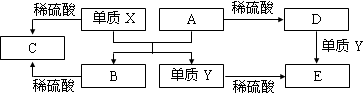
Fe2O3 + 2Al ��ʵ������ת����ϵ���� ����C����ˮ��õ�ǿ����Һ����A������Na ����C����Һ��Na2CO3���ų�CO2���壬��A������H2 ������C����Һ�еμ�NaOH��Һֱ�������������ɰ�ɫ������֮��������ܽ⣬��B����ΪA1 ����C����Һ�еμ�NaOH��Һ����ɫ�������ɣ���B����ΪCu
��ʵ������ת����ϵ���� ����C����ˮ��õ�ǿ����Һ����A������Na ����C����Һ��Na2CO3���ų�CO2���壬��A������H2 ������C����Һ�еμ�NaOH��Һֱ�������������ɰ�ɫ������֮��������ܽ⣬��B����ΪA1 ����C����Һ�еμ�NaOH��Һ����ɫ�������ɣ���B����ΪCu