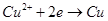��Ŀ����
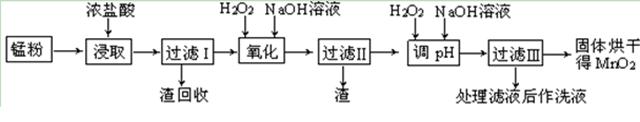
���յķϾ�п�̸ɵ�ؾ���������õ��̷�(��MnO2��Mn(OH)2��Fe����Ȳ�ͺ�̿��)�����̷���ȡMnO2�IJ�������ͼ��ʾ��
������ͼ��ʾ���貢�ο��������ݣ��ش��������⡣
��1���ڼ�����������Ũ�����ȡ�̷ۣ�������Һ�к���Mn2+��Fe2+�ȡ�MnO2��Ũ���ᷴӦ�����ӷ��̷���ʽ�� _��
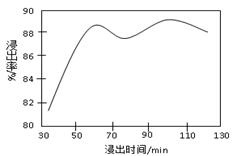
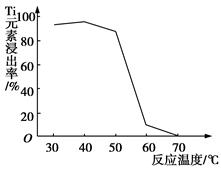
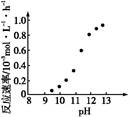
��2�����ʱ������ʱ����̽����ʵ�Ӱ������ͼ��ʾ����ҵ���õ��ǽ�ȡ60 min�������ԭ���� ��
��3���̷۾�Ũ�����ȡ������I��ȥ�������ʺ�����Һ�м�������H2O2��Һ���������� ��
��4������I������Һ�������������NaOH��Һ����pHԼΪ5.1����Ŀ���� ��
��5�����ˢ�������Һ��������H2O2��Һ������NaOH��Һ����pHԼΪ9��ʹMn2+ �����õ�MnO2����Ӧ�����ڷ���ʽΪ ��
��6����ҵ������KOH��MnO2Ϊԭ����ȡKMnO4����Ҫ�������̷��������У���һ����MnO2����KOH���飬��Ͼ��ȣ��ڿ����м������ۻ�����������������ȡK2MnO4���ڶ���Ϊ���K2MnO4��Ũ��Һ��ȡKMnO4��
�� ��һ����Ӧ�Ļ�ѧ����ʽΪ ��
�� ���K2MnO4��Ũ��Һʱ��������������ʵ������Ϊ ��

������ͼ��ʾ���貢�ο��������ݣ��ش��������⡣
| �� �� | ��ʼ���� | ������ȫ |
| Fe(OH)3 | 2.7 | 3.7 |
| Fe(OH)2 | 7.6 | 9.6 |
| Mn(OH)2 | 8.3 | 9.8 |
��1���ڼ�����������Ũ�����ȡ�̷ۣ�������Һ�к���Mn2+��Fe2+�ȡ�MnO2��Ũ���ᷴӦ�����ӷ��̷���ʽ�� _��
��2�����ʱ������ʱ����̽����ʵ�Ӱ������ͼ��ʾ����ҵ���õ��ǽ�ȡ60 min�������ԭ���� ��

��3���̷۾�Ũ�����ȡ������I��ȥ�������ʺ�����Һ�м�������H2O2��Һ���������� ��
��4������I������Һ�������������NaOH��Һ����pHԼΪ5.1����Ŀ���� ��
��5�����ˢ�������Һ��������H2O2��Һ������NaOH��Һ����pHԼΪ9��ʹMn2+ �����õ�MnO2����Ӧ�����ڷ���ʽΪ ��
��6����ҵ������KOH��MnO2Ϊԭ����ȡKMnO4����Ҫ�������̷��������У���һ����MnO2����KOH���飬��Ͼ��ȣ��ڿ����м������ۻ�����������������ȡK2MnO4���ڶ���Ϊ���K2MnO4��Ũ��Һ��ȡKMnO4��
�� ��һ����Ӧ�Ļ�ѧ����ʽΪ ��
�� ���K2MnO4��Ũ��Һʱ��������������ʵ������Ϊ ��
��1��MnO2 + 4H+ + 2Cl- =Mn2+ + Cl2��+ 2H2O(3��)
��2��60min�����ӳ�����ʱ�䣬���������ɱ������������������ԣ�(2�֣�
��3����Fe2+ת��ΪFe3+(2�֣�
��4��ʹFe3+��ȫת��ΪFe(OH)3����������ֹMn2+���Mn(OH)2(2��)
��5��Mn2+ + H2O2 + 2OH- = MnO2��+2H2O(3��)
��6��2MnO2 + 4KOH + O2= 2K2MnO4 + 2H2O(2��)��������ɫ����(2��)
��2��60min�����ӳ�����ʱ�䣬���������ɱ������������������ԣ�(2�֣�
��3����Fe2+ת��ΪFe3+(2�֣�
��4��ʹFe3+��ȫת��ΪFe(OH)3����������ֹMn2+���Mn(OH)2(2��)
��5��Mn2+ + H2O2 + 2OH- = MnO2��+2H2O(3��)
��6��2MnO2 + 4KOH + O2= 2K2MnO4 + 2H2O(2��)��������ɫ����(2��)
�����������1����Ũ�����ȡ�̷ۺ�������Һ����Mn2+ ������MnO2��Ũ���ᷢ����������ԭ��Ӧ�����ѧ����ʵ������������ʵ���֪MnO2��Ũ���ᷴӦ�����ӷ���ʽΪMnO2 + 4H+ + 2Cl- =Mn2+ + Cl2��+ 2H2O��
��2���ӽ����ʺ�ʱ��ͼ����Կ�������ʼ����ʱ������������Ӧ������60min������ʿ�ʼ�½�����������������ǽ�������60minʱ��ȱ仯�������Թ�ҵ��ȡ�Ľ���ʱ�������60min����������ʹ�����ʽϸߣ����ܽ�Լ�ɱ���
��3������I��ȥ�������ʺ���Һ�к��д���Fe2+ ���ʣ����Լ�������H2O2��ҺʹFe2+ ת��Fe3+ �Ӷ���ȫ������ȥ��
��4����ΪFe3+ ��ȫ������pHֵΪ3.7������Һ��Mn2+ ��ʼ������pHΪ8.3�����Գ���Fe3+��ͬʱ������Mn2+ ����������Ҫ����pHֵԼΪ5.1��
��5����������ԭ��Ӧ���ӷ���ʽ��дҪע�ⷴӦ�Ļ���Ϊ���Ի�������OH-�μӣ������жϳ��������ͻ�ԭ���Լ�������ѵó����ӷ���ʽΪMn2+ + H2O2 + 2OH- = MnO2��+2H2O��
��6���Ʊ�������ص�һ���У�Mn�Ļ��ϼ���+4��+6�ۣ�������������MnO2 ����ԭ������Ӧ�����������뷴Ӧ�����Ը��ݵ�ʧ�����غ���ƽ�ķ���ʽΪ��2MnO2 + 4KOH + O2= 2K2MnO4 + 2H2O��

��ϰ��ϵ�д�
�����Ŀ
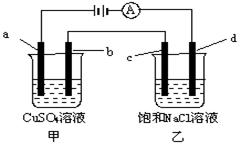
 Cu2++H2��,�����й��ڸ�װ�õ��й�˵������ȷ����(����)
Cu2++H2��,�����й��ڸ�װ�õ��й�˵������ȷ����(����)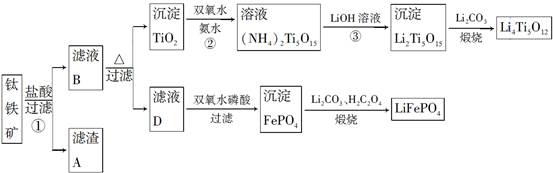
 Li7Ti5O12��3FePO4���õ�س��ʱ������Ӧʽ��____________________��
Li7Ti5O12��3FePO4���õ�س��ʱ������Ӧʽ��____________________��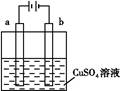

 �Ļ����Һ������������Һ��pHֵ��ʱ��t�仯��ʾ��ͼ����ʾ�������ǵ����������ˮ�ķ�Ӧ�����Է���������������ȷ����
�Ļ����Һ������������Һ��pHֵ��ʱ��t�仯��ʾ��ͼ����ʾ�������ǵ����������ˮ�ķ�Ӧ�����Է���������������ȷ����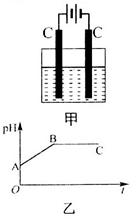
 ������A����Һ��pHֵС��B��
������A����Һ��pHֵС��B��