��Ŀ����
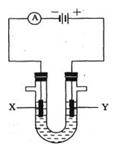
��װ���������������ʵ���֮��Ϊ1��2��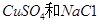 �Ļ����Һ������������Һ��pHֵ��ʱ��t�仯��ʾ��ͼ����ʾ�������ǵ����������ˮ�ķ�Ӧ�����Է���������������ȷ����
�Ļ����Һ������������Һ��pHֵ��ʱ��t�仯��ʾ��ͼ����ʾ�������ǵ����������ˮ�ķ�Ӧ�����Է���������������ȷ����
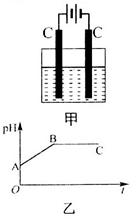
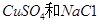 �Ļ����Һ������������Һ��pHֵ��ʱ��t�仯��ʾ��ͼ����ʾ�������ǵ����������ˮ�ķ�Ӧ�����Է���������������ȷ����
�Ļ����Һ������������Һ��pHֵ��ʱ��t�仯��ʾ��ͼ����ʾ�������ǵ����������ˮ�ķ�Ӧ�����Է���������������ȷ����
A���Ǹû����Һ�е� ������A����Һ��pHֵС��B�� ������A����Һ��pHֵС��B�� |
B��AB�߶���BC�߶��������Ϸ����ķ�Ӧ����ͬ�ļ���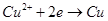 |
| C��BC�����������������������֮��Ϊ2��1 |
| D�����������Ĺ����л������������ɫ��Cu��OH��2���� |
C
���������A��AB��������ӦΪ��2Cl? - 2e? =Cl2����������ӦΪ��Cu2+ +2e? =Cu����ΪCu2+Ũ�ȼ�С��ˮ�������H+Ũ�ȼ�С������B��pH����A�㣬����B��BC�������Ϸ�ӦΪ��2H+ +2e? =H2������AB��������Ӧ��ͬ������C��BC����Һ������ΪNa2SO4�����ʱˮ����⣬��������ΪH2����������ΪO2�����֮��Ϊ2:1����ȷ��D�����ʱ��Һ��pH��7���������Cu(OH)2����������

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
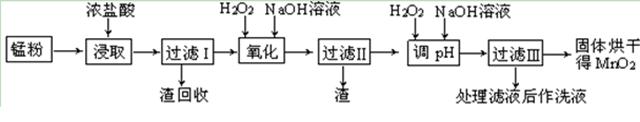
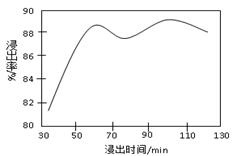
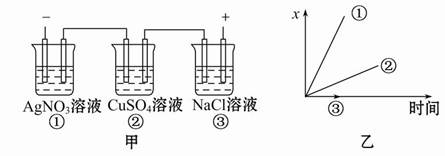
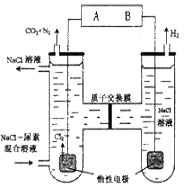


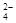 �����ϸߣ��������ӱ�ʽ����ȥSO
�����ϸߣ��������ӱ�ʽ����ȥSO