��Ŀ����
5�� X��Y��Z��W��R��T��Ԫ�����ڱ�ǰ�����ڳ���Ԫ�أ���ԭ���������������������Ϣ���±���
X��Y��Z��W��R��T��Ԫ�����ڱ�ǰ�����ڳ���Ԫ�أ���ԭ���������������������Ϣ���±���| Ԫ�� | �����Ϣ |
| X | Ԫ���������������۵ľ���ֵ��� |
| Y | �ǵؿ��к�������Ԫ�� |
| Z | Ԫ�ػ�̬ԭ��3p�ܼ�ֻ��һ������ |
| W | ��Zλ��ͬ���ڣ�������ߵ�p �ܼ�����Ϊ����� |
| R | ����Ϊ��ɫ���壬�������ڻ�ɽ�緢�� |
| T | Ԫ�ػ�̬ԭ�Ӻ�δ�ɶԵ������ |
��1��Rλ��Ԫ�����ڱ��������ڵڢ�A�壻T�Ļ�̬ԭ�Ӻ�������Ų�ʽΪ1s22s22p63s23p63d54s1��
��2���á�����������գ�
| ��һ������ | ���Ӱ뾶 | ���ӻ�ԭ�� | �����������۵� |
| W��R | Y��Z | Y��R | X��Z |
 ����
���� ����
������4��Y����ͬ����Ԫ�ص��⻯��е�仯������ͼ��ʾ������Y���⻯��е���ߵ�ԭ����ˮ���Ӽ�����γ����������ǿ�˷��Ӽ����������Ӷ�ʹˮ�ķе���ߣ�
��5����101KPa��25���£������ȶ���������1mol�û������ǵ���ЧӦ��Ϊ�û�����ı������ʣ���H������֪Z2O3�������ʣ���H��Ϊ����H=-399.09kJ/mol��T2O3�ı������ʣ���H��Ϊ����H=-269.70kJ/mol����д������Z��T2O3��Ӧ���Ȼ�ѧ����ʽ2Al��s��+Cr2O3��s��=2Cr+Al2O3��s����H=-129.39kJ/mol��
���� XԪ���������������۵ľ���ֵ��ȣ���XΪH���A��Ԫ�أ���ϣ�3����֪XΪCԪ�أ�
Y�ǵؿ��к�������Ԫ�أ���YΪOԪ�أ�ZԪ��ԭ��3p�ܼ�����1�����ӣ���Χ�����Ų�Ϊ3s23p1����ZΪAlԪ�أ�
W��Zλ��ͬ���ڣ�������ߵ�p�ܼ�����Ϊ��������������������Ų�ʽΪ��3s23p3����WΪPԪ�أ�
R�ĵ���Ϊ��ɫ���壬�������ڻ�ɽ�緢�ڣ���RΪSԪ�أ�
TԪ�ػ�̬ԭ�Ӻ�δ�ɶԵ�����࣬������Ų�ʽΪ��1s22s22p63s23p63d54s1����TΪCrԪ�أ��ݴ˽��н��
��� �⣺XԪ���������������۵ľ���ֵ��ȣ���XΪH���A��Ԫ�أ���ϣ�3����֪XΪCԪ�أ�Y�ǵؿ��к�������Ԫ�أ���YΪOԪ�أ�ZԪ��ԭ��3p�ܼ�����1�����ӣ���Χ�����Ų�Ϊ3s23p1����ZΪAlԪ�أ�W��Zλ��ͬ���ڣ�������ߵ�p�ܼ�����Ϊ��������������������Ų�ʽΪ��3s23p3����WΪPԪ�أ�R�ĵ���Ϊ��ɫ���壬�������ڻ�ɽ�緢�ڣ���RΪSԪ�أ�TԪ�ػ�̬ԭ�Ӻ�δ�ɶԵ�����࣬������Ų�ʽΪ��1s22s22p63s23p63d54s1����TΪCrԪ�أ�
��1��RΪSԪ�أ�ԭ������Ϊ16��λ��Ԫ�����ڱ��������ڵڢ�A�壻TΪCrԪ�أ����̬ԭ�Ӻ�������Ų�ʽΪ��1s22s22p63s23p63d54s1��
�ʴ�Ϊ��������A��1s22s22p63s23p63d54s1��
��2��WΪP��RΪS�����ߴ���ͬһ���ڣ�һ�����������ԭ�������ĵ���Ԫ�صĵ�һ��������������PԪ�ص�3p������Ӵ��ڰ���״̬���Ƚ��ȶ������һ�����ܴ���S��
Y����Ϊ�����ӡ�Z����Ϊ�����ӣ����ӵĵ��Ӳ���ͬʱ���˵����Խ�����Ӱ뾶ԽС���������Ӱ뾶���������ӣ�
Y����Ϊ�����ӣ�R����Ϊ�����ӣ��ǽ�����Խǿ����Ӧ���ӵĻ�ԭ��Խ�����������ӵĻ�ԭ��С�������ӣ�
X�����������Ϊ������̼��������̼Ϊ���ۻ����Z�����������Ϊ��������������Ϊ���ӻ�������Զ�����̼���۵�С����������
�ʴ�Ϊ������������������
��3��XΪC�ĵ���ΪC��RΪSԪ�أ�������������Ӧˮ�����Ũ��ҺΪŨ���ᣬC��Ũ�����ڼ��������£�����������ԭ��Ӧ���ɶ�����̼�����������ˮ����Ӧ�ķ���ʽΪ��C+2H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$CO2��+2SO2��+2H2O���������ת�Ƶķ������ĿΪ�� ��
�� ��
��
�ʴ�Ϊ�� ����
���� ����
����
��4��YΪ��Ԫ�أ���Ԫ�ص��⻯��Ϊˮ��ˮ���Ӽ�����γ����������ǿ�˷��Ӽ����������Ӷ�ʹˮ�ķе���ߣ�
�ʴ�Ϊ��ˮ���Ӽ�����γ����������ǿ�˷��Ӽ����������Ӷ�ʹˮ�ķе���ߣ�
��5��Z2O3ΪAl2O3��T2O3ΪCr2O3��Al2O3�������ʣ���H��Ϊ����H=-399.09kJ/mol�����Ȼ�ѧ����ʽΪ����2Al��s��+$\frac{3}{2}$O2��g��=Al2O3��s����H=-399.09kJ/mol��
Cr2O3�ı������ʣ���H��Ϊ����H=-269.70kJ/mol���Ȼ�ѧ����ʽΪ����2Cr��s��+$\frac{3}{2}$O2��g��=Cr2O3��s����H=-269.70kJ/mol��
���ݸ�˹���ɢ�-�ڿɵã�����Al��Cr2O3��Ӧ���Ȼ�ѧ����ʽΪ��2Al��s��+Cr2O3��s��=2Cr+Al2O3��s����H=-129.39kJ/mol��
�ʴ�Ϊ��2Al��s��+Cr2O3��s��=2Cr+Al2O3��s����H=-129.39kJ/mol��
���� ���⿼����λ�á��ṹ�����ʹ�ϵ���ۺ�Ӧ�ã���Ŀ�Ѷ��еȣ���ȷ�ƶϸ�Ԫ������Ϊ���ؼ�������֪ʶ��϶ࡢ�ۺ��Խ�ǿ����ֿ�����ѧ���ﵽ�������������������Ӧ�û���֪ʶ��������ע����������ԭ�ӽṹ��Ԫ�����ڱ���Ԫ�������ɵĹ�ϵ��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�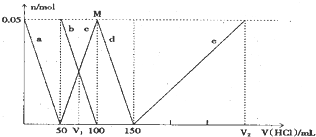
| A�� | ԭ�����Һ�е�CO32-��AlO2-�����ʵ���֮��Ϊ1��2 | |
| B�� | V1��V2=l��5 | |
| C�� | M��ʱ���ɵ�CO2Ϊ0.05mol | |
| D�� | e���߱�ʾ�����ӷ���ʽΪ��Al��OH��3+3H+=Al3++3H2O |
| A�� | NH4+��Fe3+��SO42-��Cl- | B�� | Ba2+��K+��OH-��NO3- | ||
| C�� | Al3+��Cu2+��SO42-��Cl- | D�� | Na+��Ca2+��Cl-��NO3- |
| A�� | K+��NH4+��HCO3-��AlO2- | B�� | K+��Cl-��Fe3+��SO42- | ||
| C�� | K+��I-��ClO-��Na+ | D�� | Na+��SO32-��S2-��Cl- |
| A�� | ����V2O5�������ȷ�Ӧ | B�� | þ��Ͷ�뵽FeCl3��Һ�� | ||
| C�� | ̼����CuO��������Թ��м��� | D�� | ˮ����ͨ������Na2O2��ĩ |
| A�� | Al 1s22s22p63s23p1 | B�� | O2-��1s22s22p4 | ||
| C�� | Na��1s22s22p63s1 | D�� | F��1s22s22p5 |
 ����Ӧ�úʹ������Ļ��������������������Ҫ���壮
����Ӧ�úʹ������Ļ��������������������Ҫ���壮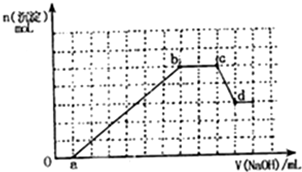 ij��ɫ��Һ�п��ܺ���H+��NH4+��Mg2+��Fe3+��Al3+��SO42-��HCO3-�����ӣ�������Һ�м���һ��Ũ��NaOH��Һ�����ɳ������ʵ��������NaOH��Һ�����ϵ��ͼ��ʾ��
ij��ɫ��Һ�п��ܺ���H+��NH4+��Mg2+��Fe3+��Al3+��SO42-��HCO3-�����ӣ�������Һ�м���һ��Ũ��NaOH��Һ�����ɳ������ʵ��������NaOH��Һ�����ϵ��ͼ��ʾ��