��Ŀ����
��H2O2��H2SO4�Ļ����Һ���ܳ��Ͼ�ӡˢ��·���ϵ�ͭ����֪��
Cu(s)+2H+(aq)=Cu2+(aq)+H2(g) ��H=+64.39kJ/mol
2H2O2(l)=2H2O(l)+O2(g) ��H=-196.46kJ/mol
H2(g)+ O2(g)=H2O(l) ��H=-285.84kJ/mol
O2(g)=H2O(l) ��H=-285.84kJ/mol
��H2SO4��Һ�У�Cu��H2O2��Ӧ����Cu2+(aq)��H2O(l)�ķ�Ӧ�Ȧ�H����
| A��-417.91kJ��mol-1 | B��-319.68 kJ��mol-1 |
| C��+546.69 kJ��mol-1 | D��-448.46 kJ��mol-1 |
B
����

��ϰ��ϵ�д�
 ������������ϵ�д�
������������ϵ�д�
�����Ŀ
Ϊ�˲ⶨ����кͷ�Ӧ���к��ȣ�����ʱ������Ҫ��������
�����Ũ�Ⱥ���� �ڼ��Ũ�Ⱥ���� �۱����� �ܷ�Ӧ����Һ������
������ˮ�����ʵ��� ��Ӧǰ����Һ�¶ȱ仯 �߲��������ʱ��
| A���٢ڢۢ� | B���٢ۢܢ� | C���ۢܢݢ� | D��ȫ�� |
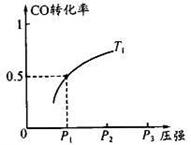
ijһ��ѧ��Ӧ�ڲ�ͬ�����µ������仯������ͼ��ʾ������˵����ȷ����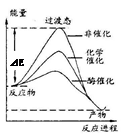
| A����ѧ����ø����Ч���� |
| B��ʹ�ò�ͬ�������Ըı䷴Ӧ����ЧӦ |
| C��ʹ�ò�ͬ�������Ըı䷴Ӧ���ܺ� |
| D����Ӧ�������������������������� |
��ͼΪijϩ���ڴ��������·����ӳɷ�Ӧ�������仯ͼ�������й������������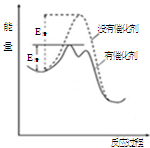
| A�������ܽ��÷�Ӧ�Ļ�� |
| B�������ܸı�÷�Ӧ���ʱ� |
| C���÷�ӦΪ���ȷ�Ӧ |
| D����ͼ��֪���ڴ��������£��÷�Ӧ����һ����ɵ� |
���շ�ӦBr + H2 HBr +H�������Է�Ӧ���̵�ʾ��ͼ�����ж���������Ӧ��������ȷ��
HBr +H�������Է�Ӧ���̵�ʾ��ͼ�����ж���������Ӧ��������ȷ��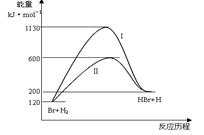
| A����Ӧ�ȣ�����I>���̢� |
| B����Ӧ���ʣ����̢�=����I |
| C���÷�ӦΪ���ȷ�Ӧ |
| D������Iʹ���˴��� |
�����й��������жϻ��ʾ������ȷ����
| A����C(ʯī)��C(���ʯ)��H��1.9 kJ��mol��1����֪���ʯ��ʯī���ȶ� |
| B�����������������������ֱ���ȫȼ�գ����߷ų��������� |
| C����H+(aq)��OH��(aq)��H2O(l) ��H��-57.3 kJ��mol��1������0.1 mol HCl�������м���4.0 gNaOH���壬�ų���������5.73 kJ |
| D��2 gH2��ȫȼ������Һ̬ˮ�ų�285.8 kJ������������ȼ�յ��Ȼ�ѧ����ʽΪ�� |
����˵���������
| A����ѧ��Ӧ���������µ�������,�������������ı仯 |
| B�����ʵ�ȼ��һ���Ƿ��ȷ�Ӧ |
| C�����ȵĻ�ѧ��Ӧ����Ҫ���Ⱦ��ܷ��� |
| D����ѧ�������֮������ת�� |
���������Ȼ�ѧ����ʽ�ó��Ľ�����ȷ����
| A����֪2H2(g)��O2(g)=2H2O(g)��H=��483.6 kJ��mol��1����������ȼ���ȣ���H��Ϊ��241.8 kJ��mol��1 |
| B����֪NaOH(aq)��HCl(aq)=NaCl(aq)��H2O(l)��H=��57.3 kJ��mol��1����40.0g NaOH��ϡ��Һ��ϡ������ȫ�кͣ��ų�С��57.3kJ������ |
| C����֪2C(s)��2O2(g)=2CO2(g)��H = a��2C(s)��O2(g)=2CO(g)����H = b����a��b |
| D����֪C (ʯī��s)="C" (���ʯ��s)��H��0����ʯī�Ƚ��ʯ�ȶ� |