��Ŀ����
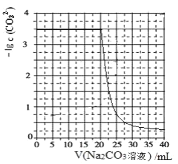
����Ŀ��ˮ���к��е� CaSO4�������� Na2CO3 ��Һ������ʹ֮ת��Ϊ���ɡ���������� CaCO3��ij��ѧ�� ȤС����ijŨ�ȵ� Na2CO3 ��Һ����һ������ CaSO4 ���壬������� Na2CO3 ��Һ�������Һ��-lgc(CO32-) �Ĺ�ϵ��ͼ��ʾ����֪Ksp(CaSO4)=9��10��6��Ksp(CaCO3)=3��10��9��lg3=0.5������˵������ȷ����
A.�����ϸ������Һ�����ϵʽ��c(Ca2��)��c(SO42��)=Ksp(CaSO4)
B.CaSO4(s)��CO32��(aq) ![]() CaCO3(s)��SO42��(aq) K=3��103
CaCO3(s)��SO42��(aq) K=3��103
C.��Na2CO3��Һ��Ũ��Ϊ1.5mol��L��1
D.��ͬ�����£�����Na2CO3��Һ��Ũ�ȸ�ΪԭŨ�ȵ�2��������ͼ������������ƽ��1����λ����
���𰸡�CD
��������
A�� CaSO4���ܶȻ�����ʽΪKsp(CaSO4)= c(Ca2��)��c(SO42��)����A��ȷ��
B��CaSO4(s)��CO32��(aq) ![]() CaCO3(s)��SO42��(aq)�Ļ�ѧƽ�ⳣ��
CaCO3(s)��SO42��(aq)�Ļ�ѧƽ�ⳣ��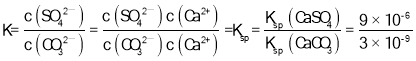 =3��103����B��ȷ��
=3��103����B��ȷ��
C����������B�������֪��CaSO4(s)��CO32��(aq) ![]() CaCO3(s)��SO42��(aq) K=3��103��ÿ����1mol CO32��������1mol SO42������CaSO4��ȫ�ܽ�ǰ��Һ�е�c(SO42��) ����Na2CO3��Һ��Ũ�ȣ���Na2CO3��ҺŨ��Ϊ1.5mol/L����c(SO42��)= 1.5mol/L ������
CaCO3(s)��SO42��(aq) K=3��103��ÿ����1mol CO32��������1mol SO42������CaSO4��ȫ�ܽ�ǰ��Һ�е�c(SO42��) ����Na2CO3��Һ��Ũ�ȣ���Na2CO3��ҺŨ��Ϊ1.5mol/L����c(SO42��)= 1.5mol/L ������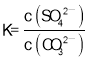 =3��103�ɵ���Һ��c(CO32��)=
=3��103�ɵ���Һ��c(CO32��)=![]() ��-lgc(CO32��)=3.3����ͼ���������C����
��-lgc(CO32��)=3.3����ͼ���������C����
D�����������֪�����CaSO4�����ʵ���Ϊ0.02mol������ͬʵ�������£�����Na2CO3��ҺŨ�ȸ�Ϊԭ����2����������̼���Ƶ����Ϊ10mL����CaSO4��ȫ�ܽ�ǰ��Һ�е�c(SO42��)=2mol/L������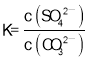 =3��103�ɵ���Һ��c(CO32��)=
=3��103�ɵ���Һ��c(CO32��)= ![]() ������-lgc(CO32��)=3.2��ͼ���еĺ������궼Ҫ�ı䣬��D����
������-lgc(CO32��)=3.2��ͼ���еĺ������궼Ҫ�ı䣬��D����
��ѡCD��

 ���Ž�������С״Ԫϵ�д�
���Ž�������С״Ԫϵ�д�����Ŀ���Ը�����(��Ҫ�ɷ�ΪFeO��Cr2O3������Al2O3��SiO2������)Ϊ��Ҫԭ����������ԭ�Ϻ췯��(��Ҫ�ɷ�Na2Cr2O7��2H2O)������Ҫ�����������£�

�������ϵ�֪:
���������£�NaBiO3������ˮ����ǿ�����ԣ��ڼ��������£��ܽ�Cr3+ת��ΪCrO42����
����
�������� | Fe3+ | Al3+ | Cr3+ | Fe2+ | Bi3+ |
��ʼ������pH | 2.7 | 3.4 | 5.0 | 7.5 | 0.7 |
������ȫ��pH | 3.7 | 4.9 | 5.9 | 9.7 | 4.5 |
�ش��������⣺
��1����Ӧ֮ǰ�Ƚ���ʯ�����Ŀ����__________________��
��2������ۼӵ��Լ�Ϊ_____________����ʱ��ҺpHҪ����5��Ŀ��_______________ ��
��3��д����Ӧ�ܵ����ӷ�Ӧ����ʽ______________________��
��4�������ữ��ʹCrO42��ת��ΪCr2O72����д���÷�Ӧ�����ӷ���ʽ_________________��
��5������ҺH��������Ũ������ȴ�ᾧ�����ˣ�ϴ�ӣ����T�ú췯�ƴ־��壬���ƺ췯����Դ־�����Ҫ���õIJ�����__________________(���������)��
����Ŀ��I.��֪���� NaHCO3(s)==Na+(ag)+HCO3��(aq) ��H=+18.81kJ��mo1��1
��Na2CO3(s)==2Na+(aq)+CO32-(aq)��H=��16.44 k J�� mol��1
��2NaHCO3(s)==Na2CO3(s)+CO2(g)+H2O(1) ��H=+92.34kJ��mol��1
��ش�
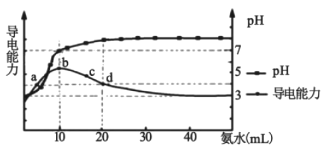
(1)������ʾ��NaHCO3������ȵ�100�淢���ֽ⣬���Ǽ��� NaHCO3��Һ����80����д���CO2����ų����÷�Ӧ�ȽǶ�˵��ԭ��_________________________________��
(2) NaHCO3��Һ����Ҫ����2�ֻ�ѧƽ�⣺a.HCO3��+H2O![]() H2CO3+OH����b.2HCO3��
H2CO3+OH����b.2HCO3��![]() CO32��+H2O+CO2���������ۼ���0.10 mol��.L��1 NaHCO3��Һ��2����Ӧ��ת�������¶ȱ仯��ͼ��ʾ(�������Ӱ��)��
CO32��+H2O+CO2���������ۼ���0.10 mol��.L��1 NaHCO3��Һ��2����Ӧ��ת�������¶ȱ仯��ͼ��ʾ(�������Ӱ��)��

�ټ���25��0.10mol��L��1NaHCO3��Һ��CO2��H2CO3����Ũ��������Ϊ___________mol��L��1��
�ڼ�������NaHCO3��Һ���õ��Ĺ�����___________��
��25��ʱ0.10mol��L��1��NaHCO3��ҺpH=8.3�����ȵ�4������Һ���ڣ����µ�7���ӡ���֪������Na2CO3��ҺŨ�Ⱥ�pH�Ĺ�ϵ���±�(�����¶ȶ�Kw��Ӱ��)��
c(mo1��L��1) | ���� | 0.20 | 0.10 | 0.010 | 0.0010 |
pH | 12.1 | 11.8 | 11.5 | 11.1 | 10.6 |
����ͼ������ NaHCO3��ҺpH��ʱ��仯����______________

II.�о��ó�������ֽ�ʱ����������ƽ��ʱ��ѹ(Pa)���¶�(��)�Ĺ�ϵ��ͼ

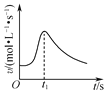
(1)T��ʱ����1L�����ܱ������г���0.3 mol CH4��ֻ������Ӧ2CH4(g)![]() C2H4(g)+2H2(g)���ﵽƽ��ʱ��c(C2H4)=c(CH4
C2H4(g)+2H2(g)���ﵽƽ��ʱ��c(C2H4)=c(CH4
(2)��ʽ���㷴Ӧ2CH4(g)![]() C2H2(g)+3H2(g)��ͼ��A���¶�ʱ��ƽ�ⳣ��K=___________(��ƽ���ѹ����ƽ��Ũ�ȼ��㣬1g0.05=��1.3)
C2H2(g)+3H2(g)��ͼ��A���¶�ʱ��ƽ�ⳣ��K=___________(��ƽ���ѹ����ƽ��Ũ�ȼ��㣬1g0.05=��1.3)
(3)��ͼ��֪�������ѽ�����Ȳ�и�������ϩ���ɣ�Ϊ���������Ȳ��ת���ʣ����ı��¶��⣬���ɲ�ȡ�Ĵ�ʩ��_________________________________��