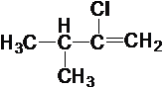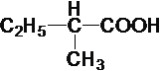ЬтФПФкШн
ЁОЬтФПЁПаЁЫеДђЁЂЮИЪцЦНЖМЪЧГЃгУЕФжаКЭЮИЫсЕФвЉЮяЃЌЭъГЩЯТСаЬюПеЃК
ЃЈ1ЃЉаЁЫеДђЦЌУПЦЌКЌ0.42gNaHCO3ЃЌ2ЦЌаЁЫеДђЦЌКЭЮИЫсЭъШЋжаКЭЃЌБЛжаКЭЕФЧтРызгЮяжЪЕФСПЪЧ__molЁЃ
ЃЈ2ЃЉЮИЪцЦНУПЦЌКЌ0.195gAl(OH)3ЃЌжаКЭЮИЫсЪБЃЌ3ЦЌаЁЫеДђЦЌЯрЕБгкЮИЪцЦН__ЦЌЁЃ
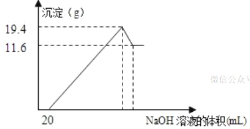
ЃЈ3ЃЉНЋвЛЖЈжЪСПЕФУОТСКЯН№ЭЖШы100mLвЛЖЈЮяжЪЕФСПХЈЖШЕФбЮЫсжаЃЌКЯН№ШЋВПШмНтЃЌЯђЫљЕУШмвКжаЕЮМг5mol/LЕФNaOHШмвКЕНЙ§СПЃЌЩњГЩГСЕэЕФжЪСПгыМгШыЕФNaOHШмвКЕФЬхЛ§ЙиЯЕШчЭМЫљЪОЁЃгЩЭМжаЪ§ОнМЦЫуЃК
ЂйдКЯН№жаУОКЭТСЕФжЪСП___ЁЂ___ЁЃ
ЂкбЮЫсЕФЮяжЪЕФСПХЈЖШ___ЁЃ
ЁОД№АИЁП0.01 2 4.8g 2.7g 8mol/L
ЁОНтЮіЁП
(1)ИљОнЬМЫсЧтФЦЕФжЪСПЧѓТШЛЏЧтЕФЮяжЪЕФСПЃЛ
(2)ИљОнЗНГЬЪНевГіЧтбѕЛЏТСгыЬМЫсЧтФЦжЎМфЕФЙиЯЕЃЛ
(3)гЩЭМПЩжЊЃЌДгПЊЪМжСМгШыNaOHШмвК20mLЃЌУЛгаГСЕэЩњГЩЃЌЫЕУїдШмвКжабЮЫсШмНтMgЁЂAlКѓгаЪЃгрЃЌДЫЪБЗЂЩњЕФЗДгІЮЊЃКHCl+NaOH=NaCl+H2OЃЎМЬајЕЮМгNaOHШмвКЃЌЕНГСЕэСПзюДѓЃЌДЫЪБЮЊMg(OH)2КЭAl(OH)3ЃЌЖўепжЪСПжЎКЭЮЊ19.4gЃЌШмвКЮЊТШЛЏФЦШмвКЃЎдйМЬајЕЮМгNaOHШмвКЃЌГСЕэСППЊЪММѕаЁЃЌГСЕэСПзюаЁЪБЮЊMg(OH)2ЃЌЦфжЪСПЮЊ11.6gЃЌЙЪЕНГСЕэСПзюДѓЪБAl(OH)3ЕФжЪСПЮЊ19.4g-11.6g=7.8gЃЛ
ЂйгЩдЊЫиЪиКуПЩжЊЃЌn(Al)=n[Al(OH)3]ЃЌn(Mg)=n[Mg(OH)2]ЃЌдйРћгУm=nMМЦЫуAlЁЂMgЕФжЪСПЃЛ
ЂкМгШы20mLNaOHШмвКЃЌЧЁКУжаКЭЪЃгрЕФбЮЫсЃЌДЫЪБШмвКжаШмжЪЮЊAlCl3ЁЂMgCl2ЁЂNaClЃЌИљОнТШдЊЫиЪиКугаn(HCl)=3n(AlCl3)+2n(MgCl2)+n(NaCl)ЃЌИљОнФЦдЊЫиЪиКуДЫЪБШмвКжаn(NaCl)=n(NaOH)ЃЌОнДЫМЦЫуГіn(HCl)ЃЌдйРћгУc=![]() МЦЫубЮЫсЕФЮяжЪЕФСПХЈЖШЁЃ
МЦЫубЮЫсЕФЮяжЪЕФСПХЈЖШЁЃ
(1)ЩшБЛжаКЭЕФHClЕФЮяжЪЕФСПЮЊxmolЃЌ
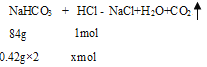
дђЃК![]() =
=![]() ЃЌНтЕУЃКx=0.01ЃЛ
ЃЌНтЕУЃКx=0.01ЃЛ
(2)ЩшЯрЕБгкЧтбѕЛЏТСЕФЦЌЪ§ЮЊyЃЌгЩЗНГЬЪНAl(OH)3+3HCl=AlCl3+3H2OКЭNaHCO3+HClЈTNaCl+H2O+CO2ЁќЕУЙиЯЕЪНЃК
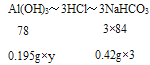
дђЃК![]() =
=![]() ЃЌНтЕУЃКy=2ЃЛ
ЃЌНтЕУЃКy=2ЃЛ
(3)гЩЭМПЩжЊЃЌДгПЊЪМжСМгШыNaOHШмвК20mLЃЌУЛгаГСЕэЩњГЩЃЌЫЕУїдШмвКжабЮЫсШмНтMgЁЂAlКѓгаЪЃгрЃЌДЫЪБЗЂЩњЕФЗДгІЮЊЃКHCl+NaOH=NaCl+H2OЁЃМЬајЕЮМгNaOHШмвКЃЌЕНГСЕэСПзюДѓЃЌДЫЪБЮЊMg(OH)2КЭAl(OH)3ЃЌЖўепжЪСПжЎКЭЮЊ19.4gЃЌШмвКЮЊТШЛЏФЦШмвКЃЎдйМЬајЕЮМгNaOHШмвКЃЌГСЕэСППЊЪММѕаЁЃЌГСЕэСПзюаЁЪБЮЊMg(OH)2ЃЌЦфжЪСПЮЊ11.6gЃЌЙЪЕНГСЕэСПзюДѓЪБAl(OH)3ЕФжЪСПЮЊ19.4g-11.6g=7.8gЁЃ
ЂйгЩдЊЫиЪиКуПЩжЊЃЌn(Al)=n[Al(OH)3]=![]() =0.1molЃЌЫљвдm(Al)=0.1molЁС27g/mol=2.7gЃЛ
=0.1molЃЌЫљвдm(Al)=0.1molЁС27g/mol=2.7gЃЛ
n(Mg)=n[Mg(OH)2]=![]() =0.2molЃЌЫљвдm(Mg)=0.2molЁС24g/mol=4.8gЃЛ
=0.2molЃЌЫљвдm(Mg)=0.2molЁС24g/mol=4.8gЃЛ
ЂкМгШы20mLNaOHШмвКЃЌЧЁКУжаКЭЪЃгрЕФбЮЫсЃЌДЫЪБШмвКжаШмжЪЮЊAlCl3ЁЂMgCl2ЁЂNaClЃЌИљОнТШдЊЫиЪиКугаn(HCl)=3n(AlCl3)+2n(MgCl2)+n(NaCl)ЃЌИљОнФЦдЊЫиЪиКуДЫЪБШмвКжаn(NaCl)=n(NaOH)=0.02LЁС5mol/L=0.1molЃЌЫљвдn(HCl)=3n(AlCl3)+2n(MgCl2)+n(NaCl)=3ЁС0.1mol+2ЁС0.2mol+0.1mol=0.8molЃЌдбЮЫсЕФЮяжЪЕФСПХЈЖШЮЊ![]() =8mol/LЁЃ
=8mol/LЁЃ