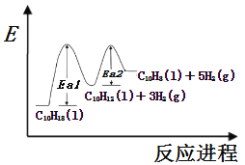
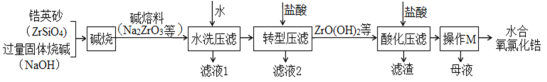
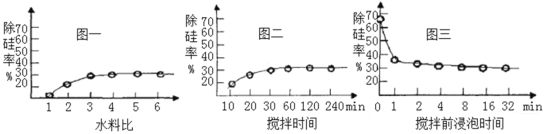
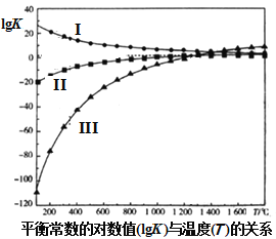
��Ŀ����
����Ŀ�����ܺϽ��ڴſء��DZ�����������Ҫ��;���ش��������⣺
(1)��̬Coԭ�Ӽ۲���ӵĹ������ʽΪ___________��Co��Ca����ͬһ���ڣ��Һ����������ӹ�����ͬ��������Co���۵�Ƚ���Ca�ĸߣ�ԭ��Ϊ________________��
(2)Pt��Cl�����(![]() )���γ���ͼ��ʾ���ַ��ӡ�
)���γ���ͼ��ʾ���ַ��ӡ�

�����������ͬ����Ԫ�صĵ�һ��������С�����˳��Ϊ________________��
��1mol��������к���![]() ������ĿΪ__________________��
������ĿΪ__________________��
����ͬ�����£����ֻ���������ˮ���ܽ�ȸ����Ϊ__(���)��ԭ��Ϊ____��
(3)ijPt-Co�Ͻ�ľ���ѻ�ģ��Ϊ���������ѻ�������Coԭ�Ӵ��ڶ���λ�á�Ptԭ�Ӵ�������λ�ã���úϽ�Ļ�ѧʽΪ_______��
(4)![]() �����ڼ���ɿ������������ӵ����幹��Ϊ__��̼ԭ�ӵ��ӻ���ʽΪ____��
�����ڼ���ɿ������������ӵ����幹��Ϊ__��̼ԭ�ӵ��ӻ���ʽΪ____��
(5)![]() ��һ�ִ��Բ��ϣ��侧���ṹ��ͼ����ʾ������ͼ��ͼ����ʾ��
��һ�ִ��Բ��ϣ��侧���ṹ��ͼ����ʾ������ͼ��ͼ����ʾ��

��ԭ�����������AΪ(0��0��0)��BΪ(0.31��0.31��0)����Cԭ�ӵ��������Ϊ__��
���������ӵ�������ֵΪ![]() �������ܶ�Ϊ___
�������ܶ�Ϊ___![]() (�г��������ʽ)��
(�г��������ʽ)��
���𰸡�![]() Co��ԭ�Ӱ뾶С��Ca���۲���Ӷ���Ca���ʽ�����ǿ��Ca C<N 26
Co��ԭ�Ӱ뾶С��Ca���۲���Ӷ���Ca���ʽ�����ǿ��Ca C<N 26![]() �� ��Ϊ�Ǽ��Է��ӣ���Ϊ���Է���
�� ��Ϊ�Ǽ��Է��ӣ���Ϊ���Է��� ![]() ��
��![]() ֱ���� sp (0.69��0. 69��0)
ֱ���� sp (0.69��0. 69��0) ![]()
��������
(1)����Ԫ�صĽ�������۲���������йأ�
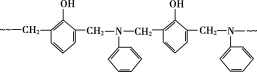
(2)ͬ���ڴ������ҵ�һ����������������ƣ���һ��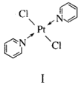 �����У�8��C-C����10��C-H����4��C-N����2��N-Pt����2��Cl-Pt�����۸����������ܹ��ɽ��ͣ�
�����У�8��C-C����10��C-H����4��C-N����2��N-Pt����2��Cl-Pt�����۸����������ܹ��ɽ��ͣ�
(3)�þ�̯��������
(4)![]() �У�SCN����CO2�ǵȵ����壬����ԭ��̼�ļ۲���Ӷ�=2+
�У�SCN����CO2�ǵȵ����壬����ԭ��̼�ļ۲���Ӷ�=2+![]() =2��û�йµ��Ӷԣ��ɴ˷�����
=2��û�йµ��Ӷԣ��ɴ˷�����
(5)���ø���ͼ���������þ�̯������ܶȹ�ʽ���㡣
(1)Co��27��Ԫ�أ��۲�����Ų�Ϊ3d74s2����̬Coԭ�Ӽ۲���ӵĹ������ʽΪ![]() ��Co��Ca����ͬһ���ڣ��Һ����������ӹ�����ͬ��������Co���۵�Ƚ���Ca�ĸߣ�ԭ��Ϊ��Co��ԭ�Ӱ뾶С��Ca���۲���Ӷ���Ca���ʽ�����ǿ��Ca���ʴ�Ϊ��
��Co��Ca����ͬһ���ڣ��Һ����������ӹ�����ͬ��������Co���۵�Ƚ���Ca�ĸߣ�ԭ��Ϊ��Co��ԭ�Ӱ뾶С��Ca���۲���Ӷ���Ca���ʽ�����ǿ��Ca���ʴ�Ϊ��![]() ��Co��ԭ�Ӱ뾶С��Ca���۲���Ӷ���Ca���ʽ�����ǿ��Ca��
��Co��ԭ�Ӱ뾶С��Ca���۲���Ӷ���Ca���ʽ�����ǿ��Ca��
(2)��![]() ����к�C��N��H��N��2p3�ǰ����״̬����һ�����ܴ�
����к�C��N��H��N��2p3�ǰ����״̬����һ�����ܴ�![]() ����ͬ����Ԫ�صĵ�һ��������С�����˳��ΪC<N���ʴ�Ϊ��C<N��
����ͬ����Ԫ�صĵ�һ��������С�����˳��ΪC<N���ʴ�Ϊ��C<N��
��һ�� �����У�8��C-C����10��C-H����4��C-N����2��N-Pt����2��Cl-Pt����1mol��������к���
�����У�8��C-C����10��C-H����4��C-N����2��N-Pt����2��Cl-Pt����1mol��������к���![]() ������ĿΪ26
������ĿΪ26![]() ���ʴ�Ϊ��26
���ʴ�Ϊ��26![]() ��
��
�۸����������ܹ��ɣ���ͬ�����£����ֻ���������ˮ���ܽ�ȸ����Ϊ��ԭ��Ϊ��Ϊ�Ǽ��Է��ӣ���Ϊ���Է��ӡ��ʴ�Ϊ����Ϊ�Ǽ��Է��ӣ���Ϊ���Է��ӣ�
(3)ijPt-Co�Ͻ�ľ���ѻ�ģ��Ϊ���������ѻ�������Coԭ�Ӵ��ڶ���λ��8��![]() =1��Ptԭ�Ӵ�������λ��6��
=1��Ptԭ�Ӵ�������λ��6��![]() =3����úϽ�Ļ�ѧʽΪ
=3����úϽ�Ļ�ѧʽΪ![]() ��
��![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��![]() ��
��
(4)![]() �У�SCN����CO2�ǵȵ����壬����ԭ��̼�ļ۲���Ӷ�=2+
�У�SCN����CO2�ǵȵ����壬����ԭ��̼�ļ۲���Ӷ�=2+![]() =2��û�йµ��Ӷԣ�SCN�������幹��Ϊֱ���Σ�̼ԭ�ӵ��ӻ���ʽΪsp���ʴ�Ϊ��ֱ���Σ�sp��
=2��û�йµ��Ӷԣ�SCN�������幹��Ϊֱ���Σ�̼ԭ�ӵ��ӻ���ʽΪsp���ʴ�Ϊ��ֱ���Σ�sp��
(5)�ٴӸ���ͼ�У�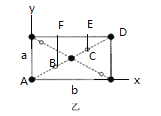 ԭ�����������AΪ(0��0��0)��BΪ(0.31��0.31��0)��A B��x��y���ϵ�ͶӰ�ֱ�Ϊ0.31b��0.31a,ͼ��AB���ȵ���CD���ȣ���AC����x��y���ϵ�ͶӰ�ֱ�Ϊ(b-0.31b)��(a-0.31a),����0.69b��0.69a,��Cԭ�ӵ��������Ϊ(0.69��0. 69��0)���ʴ�Ϊ��(0.69��0. 69��0)��
ԭ�����������AΪ(0��0��0)��BΪ(0.31��0.31��0)��A B��x��y���ϵ�ͶӰ�ֱ�Ϊ0.31b��0.31a,ͼ��AB���ȵ���CD���ȣ���AC����x��y���ϵ�ͶӰ�ֱ�Ϊ(b-0.31b)��(a-0.31a),����0.69b��0.69a,��Cԭ�ӵ��������Ϊ(0.69��0. 69��0)���ʴ�Ϊ��(0.69��0. 69��0)��
���������ӵ�������ֵΪ![]() ��Co��1+8��
��Co��1+8��![]() =2��O��2+4��
=2��O��2+4��![]() =4�������ܶ�Ϊ
=4�������ܶ�Ϊ![]()
![]() (�г��������ʽ)���ʴ�Ϊ��
(�г��������ʽ)���ʴ�Ϊ��![]() ��
��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ��Ԫ�ص��ʼ��仯�����й㷺��;����������ڱ��е�������Ԫ�����֪ʶ�ش��������⣺
��1����ԭ������������˳��ϡ��������⣩������˵����ȷ����___��
a��ԭ�Ӱ뾶�����Ӱ뾶����С b�������Լ������ǽ�������ǿ
c�����ʵ��۵㽵�� d���������Ӧ��ˮ������Լ�����������ǿ
��2��ԭ�������������������������ͬ��Ԫ������Ϊ___�������������ļ���������___��
��3����֪��
������ | MgO | Al2O3 | MgCl2 | AlCl3 |
���� | ���ӻ����� | ���ӻ����� | ���ӻ����� | ���ۻ����� |
�۵�/�� | 2800 | 2050 | 714 | 191 |
��ҵ��þʱ�����MgCl2�������MgO��ԭ����___������ʱ�����Al2O3�������AlCl3��ԭ����___��
��4������裨�۵�1410���������õİ뵼����ϣ��ɴֹ��ƴ���������£�
Si���֣�![]() SiCl4
SiCl4![]() SiCl4������
SiCl4������![]() Si������
Si������
д��SiCl4�ĵ���ʽ��___����������SiCl4�ƴ���ķ�Ӧ�У����ÿ����1.12kg����������akJ������д���÷�Ӧ���Ȼ�ѧ����ʽ___��
��5���������岻����Ũ����������P2O5�������___��
a��NH3 b��HI c��SO2 d��CO2
��6��KClO3������ʵ������O2�������Ӵ�����400��ʱ�ֽ�ֻ���������Σ�����һ�����������Σ���һ���ε��������Ӹ�����Ϊ1��1��д���÷�Ӧ�Ļ�ѧ����ʽ��___��